
Low-pressure pneumoperitoneum—why and how
Introduction
The optimal level of pressure for pneumoperitoneum has been discussed for years. Until recently, data have not been available for the effects of low versus standard pressure pneumoperitoneum on intraoperative as well as postoperative outcomes. Furthermore, there may be differences within and between specialties. However, in most institutions, there seems to be a consensus that 12 mmHg is the chosen standard pressure for laparoscopy, but data exist for the effects of pneumoperitoneum both with lower and higher pressure levels. A recent meta-analysis found significant effects of low-pressure pneumoperitoneum in laparoscopic cholecystectomy on postoperative pain and analgesic consumption (1) and we therefore found it of interest to summarize the available evidence on why to aim for the lowest possible pressure for pneumoperitoneum and how in clinical practice to reach that goal without compromising patient safety.
Why
Pain
A recent meta-analysis found that low-pressure pneumoperitoneum (6–10 mmHg) compared with standard or high-pressure pneumoperitoneum (12–15 mmHg) resulted in lower postoperative pain at 1, 4, 8, and 12 hours, and one day postoperatively, whereas the difference was not significant after two or three days (1). There was also a significant difference in postoperative shoulder pain favoring the low-pressure group, thus confirming a previous meta-analysis on this subject (2).
No harms
The meta-analysis (1) was not able to show a difference between low- and standard-pressure pneumoperitoneum on overall complication rates, bleeding, interoperative bile spillage, conversion to open surgery and length of hospital stay, meaning that the use of low-pressure pneumoperitoneum was safe. However, the previous meta-analysis did find an effect on hospital stay in favor of low-pressure pneumoperitoneum (2), but as length of stay is a subjective parameter, which is highly dependent on routines and traditions unless a rigid fast track regimen is in place, the important conclusion regarding the available data would simply be that low-pressure pneumoperitoneum is safe for the patient and that it has positive effects on pain and analgesic consumption. The meta-analysis found a significant difference in operative time favoring standard pressure laparoscopy (1) but as the difference was only around 1.5 minutes, it is without any clinical significance.
Immune function and cellular effects
A recent randomized controlled trial in patients undergoing laparoscopic colorectal surgery using low-pressure versus standard-pressure pneumoperitoneum found that the quality of recovery on postoperative day 1 was significantly higher in patients undergoing low-pressure pneumoperitoneum, and the decline in cytokine production capacity was significantly less for tumor necrosis factor-alpha and interleukin-6 for patients operated at low pressure (3). Similar results were found in a multicenter randomized clinical trial in laparoscopic colorectal surgery originating from Spain, the Netherlands, Thailand and the UK (4), where they found faster recovery, fewer intraoperative complications and less inflammation with low versus standard pneumoperitoneum pressure.
These very interesting findings of the effects of pneumoperitoneum on the immune system, as expressed in blood cytokine levels, have also been supported by a study in patients undergoing laparoscopic hysterectomy with standard (12 mmHg) versus low (8 mmHg) pneumoperitoneum, but now looking at the cellular peritoneal environment (5). This study, including a total of 68 patients, measured an array of molecular parameters from macroscopically normal peritoneum. They found that expression levels of connective tissue growth factor, matrix metalloproteinase-9, E-selectin, chemokine ligand 2, hyaluronidase-1 and -2 were significantly higher, and hyaluronic acid synthase-1 and -3, thrombospondin-2, interleukin-10, and hyaluronan synthesis were significantly lower in the 12-mmHg group compared with the 8-mmHg group (5). These findings mean that low-pressure pneumoperitoneum might minimize the adverse effects of intraperitoneal pressure on inflammation and peritoneal fibrosis (5), which could potentially be relevant for fertility because of fewer adhesions (6), however not shown in clinical outcome trials yet. Furthermore, numerous experimental studies have investigated the effects of pneumoperitoneum on the local peritoneal environment and have shown that pneumoperitoneum is associated with local and systemic inflammation, acidosis, oxidative stress, mesothelium lining abnormalities, and adhesion development (7), although we do not yet have final evidence for detrimental clinical outcome from human randomized clinical trials.
Intracranial and intraocular pressure
A study in 101 patients undergoing laparoscopic cholecystectomy randomized to either low-pressure (8 mmHg) and high-pressure (14 mmHg) pneumoperitoneum during surgery found that high-pressure surgery caused significant increase in intracranial pressure compared with low-pressure pneumoperitoneum during laparoscopic cholecystectomy (8). Although the study did not find any clinical complications because of the changes in intracranial pressure they concluded that it may be advisable to operate at low-pressure pneumoperitoneum in high-risk patients (8).
Postoperative visual loss has been reported in case reports and it has been estimated that the incidence after non-ocular surgery may be as low as 0.0002% (9). There may be many pathogenic factors behind postoperative visual loss and high-pressure pneumoperitoneum is likely to play a role in some cases (9). It has also been shown that propofol anesthesia may to some extent protect against increased intraocular pressure compared with volatile anesthetic agents (10).
Intraoperative cardiac problems
In the early years of laparoscopic surgery, there were major concerns about the intraoperative cardiovascular consequences of pneumoperitoneum (11). In those years, numerous experimental studies in animals as well as humans found that pneumoperitoneum will increase systemic vascular resistance, increase pulmonary vascular resistance, and decrease cardiac index (11). Many anesthesiologists were concerned about these data, and it happened frequently in many institutions that patients with just slight or moderate cardiac comorbidity were denied laparoscopic surgery. As years went by it was, however, obvious that clinical cardiac complications in fact did not or only very rarely occur and nowadays laparoscopic surgery is routinely offered to patients with moderate or even high cardiac risk.
In the rare situation where establishment of pneumoperitoneum triggers partial cardiovascular collapse presenting as severely decreased blood pressure not responding to pharmacological intervention it is very important to collaborate closely between surgeon and anesthesiologist (12). The first thing to do would be to decrease intraabdominal pressure to zero, and if cardiovascular function is restored then to reestablish pneumoperitoneum to a level as low as possible still maintaining adequate surgical exposure. In this very rare situation, it may be a good idea to establish deep neuromuscular blockade, alter the position of the patient, and secure high surgical expertise in order to complete the laparoscopic operation as fast as possible and under maybe suboptimal conditions. Of course, it is most often possible to convert to open surgery, but it is important to remember that postoperative complications are substantially higher with open surgery compared with minimal invasive surgery, and it is therefore advisable to try to complete the operation with the laparoscopic approach if this can be done safely and with close collaboration between surgeon and anesthesiologist.
Hepato-renal problems
Similar to the cardiovascular changes an increase in pneumoperitoneal pressure can also lead to hepato-renal modifications. It may decrease hepatic arterial and portal venous blood flow and can reduce splanchnic perfusion. Vena cava compression may decrease venous return and pooling of venous blood in the lower extremities (13). Finally, pneumoperitoneum has important effects on renal physiology. Direct compression of the renal vasculature can lead to a reduction in renal blood flow, glomerular filtration rate, and oliguria (13). Although uncommon, laparoscopy poses an increased risk of acute kidney injury in patients with preexisting kidney disease (14).
How
Several lines of evidence, and summarized in a newly published meta-analysis, suggest that low pressure peritoneum significantly reduces the incidence of mild to moderate postoperative complications, reduces early postoperative pain scores, reduces postoperative nausea and vomiting (PONV) scores and reduces the mean length of hospital stay (15). However, it remains to be determined how peritoneal pressure can be reduced without compromising surgical conditions and patient safety.
Deep block
Neuromuscular blocking agents are commonly used to facilitate tracheal intubation and to improve surgical exposure (16). Intraoperatively, the anaesthetist evaluates the depth of neuromuscular blockade at the adductor pollicis muscle (16). Unfortunately, both the diaphragm and the muscles in the abdominal wall are more resistant than the adductor pollicis muscle to neuromuscular blocking agents (17,18). Thus, these muscles may already have partly recovered whilst the adductor pollicis muscle is still completely paralyzed. To ensure that the diaphragm and the abdominal wall muscles are also completely paralyzed, deep neuromuscular blockade defined as a posttetanic count of 1 to 3 must be established (19). Interestingly in this context, a previous study hypothesized that relaxation of the diaphragm and the muscles in the abdominal wall may compensate to some degree for insufflation pressure when establishing the pneumoperitoneum (20). To prove this hypothesis the authors quantified the intraabdominal volume during laparoscopy in two groups of patients with either an insufflation pressure of 8 mmHg (“low insufflation pressure group”) or 12 mmHg (“standard insufflation pressure group”). Moreover, each group was further divided into a subgroup with deep neuromuscular blockade and a second one without any neuromuscular blockade.
In the same study, their most important findings were that at a constant degree of neuromuscular blockade higher insufflation pressure led to more intraabdominal volume (20). They also demonstrated that at a given insufflation pressure, i.e., 8 mmHg or 12 mmHg, the intraabdominal volume was significantly larger in the subgroup with a deep neuromuscular block when compared to the subgroup without neuromuscular blockade (Figure 1). Thus, optimizing the neuromuscular environment further improves surgical exposure as it provides more surgical space for a given insufflation pressure. Finally, no differences in intraabdominal volume were found between patients with high insufflation pressure (i.e., 12 mmHg) but no neuromuscular blockade and those with low insufflation pressure (i.e., 8 mmHg) and deep neuromuscular blockade. Thus, optimizing the management of neuromuscular blockade may allow to maintain surgical exposure whilst reducing intraabdominal insufflation pressure. As a consequence, this “deep block concept” may contribute to attenuate the insufflation pressure induced side effects such as postoperative (shoulder) pain and cardiovascular instability. This approach may be of particular clinical interest in the ambulatory setting and in patients with an increased cardiovascular risk such as the elderly.
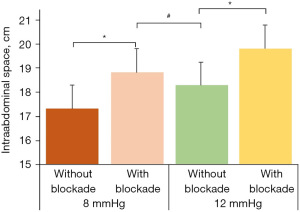
Accordingly, the recently published guideline on the peri-operative management of neuromuscular blockade from the European Society of Anaesthesiology and Intensive Care recommends deepening neuromuscular blockade if surgical conditions need to be improved (16).
Positioning
Positioning of the patient in either steep Trendelenburg or anti-Trendelenburg position is an important tool to improve surgical exposure (12). There has been, however, concern in daily clinical practice if steep Trendelenburg position would cause problems such as pulmonary complications because of decreased lung volume during the procedure, intraoperative hemodynamic/cardiac complications (21), and intracranial changes because of increased intracranial pressure (22). However, a recent systematic review and meta-analysis showed that steep Trendelenburg position does not seem to affect postoperative clinical complications (23). To our knowledge, there are no data to suggest that steep anti-Trendelenburg position has any negative effects on postoperative clinical complications either.
Expertise
There are no data available on whether operating at low intraabdominal pressure will require a certain level of surgical expertise. It is, however, possible that operating at low pressure will require some degree of habituation, and as with all new surgical adjustments they are most likely to be carried into daily clinical practice by the most technically experienced surgeons. Nevertheless, if low intraabdominal pressure is accompanied by deep neuromuscular blockade and proper patient positioning it may in fact not be a surgical challenge for the operator. We therefore suggest that collaboration between surgeons and anaesthesiologists together with increased knowledge levels in both specialties about their counterparts should be a focus area (12), which may facilitate the use of low intraabdominal pressure without being a surgical challenge.
Conclusions
Mounting evidence suggests that the use of low-pressure pneumoperitoneum can reduce postoperative pain and the need for analgesics, potentially improving patient outcomes. However, lower insufflation pressures may compromise surgical conditions. The solution may lie in deep neuromuscular blockade, which could allow for both lower insufflation pressures and an adequate surgical view.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://ls.amegroups.com/article/view/10.21037/ls-23-10/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://ls.amegroups.com/article/view/10.21037/ls-23-10/coif). The authors have previously received speakers’ fees from MSD, outside the current manuscript. JR also serves as an unpaid editorial board member of Laparoscopic Surgery from February 2022 to January 2024. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ortenzi M, Montori G, Sartori A, et al. Low-pressure versus standard-pressure pneumoperitoneum in laparoscopic cholecystectomy: a systematic review and meta-analysis of randomized controlled trials. Surg Endosc 2022;36:7092-113. [Crossref] [PubMed]
- Raval AD, Deshpande S, Koufopoulou M, et al. The impact of intra-abdominal pressure on perioperative outcomes in laparoscopic cholecystectomy: a systematic review and network meta-analysis of randomized controlled trials. Surg Endosc 2020;34:2878-90. [Crossref] [PubMed]
- Albers KI, Polat F, Helder L, et al. Quality of Recovery and Innate Immune Homeostasis in Patients Undergoing Low-pressure Versus Standard-pressure Pneumoperitoneum During Laparoscopic Colorectal Surgery (RECOVER): A Randomized Controlled Trial. Ann Surg 2022;276:e664-73. [Crossref] [PubMed]
- Díaz-Cambronero O, Mazzinari G, Flor Lorente B, et al. Effect of an individualized versus standard pneumoperitoneum pressure strategy on postoperative recovery: a randomized clinical trial in laparoscopic colorectal surgery. Br J Surg 2020;107:1605-14. [Crossref] [PubMed]
- Matsuzaki S, Jardon K, Maleysson E, et al. Impact of intraperitoneal pressure of a CO2 pneumoperitoneum on the surgical peritoneal environment. Hum Reprod 2012;27:1613-23. [Crossref] [PubMed]
- Canis M, Botchorishvili R, Bourdel N, et al. Pelvic adhesions and fertility: Where are we in 2018? J Visc Surg 2018;155:S11-5. [Crossref] [PubMed]
- Umano GR, Delehaye G, Noviello C, et al. The "Dark Side" of Pneumoperitoneum and Laparoscopy. Minim Invasive Surg 2021;2021:5564745. [Crossref] [PubMed]
- Yashwashi T, Kaman L, Kajal K, et al. Effects of low- and high-pressure carbon dioxide pneumoperitoneum on intracranial pressure during laparoscopic cholecystectomy. Surg Endosc 2020;34:4369-73. [Crossref] [PubMed]
- Ripa M, Schipa C, Kopsacheilis N, et al. The Impact of Steep Trendelenburg Position on Intraocular Pressure. J Clin Med 2022;11:2844. [Crossref] [PubMed]
- Chang CY, Chien YJ, Wu MY. Attenuation of increased intraocular pressure with propofol anesthesia: A systematic review with meta-analysis and trial sequential analysis. J Adv Res 2020;24:223-38. [Crossref] [PubMed]
- Struthers AD, Cuschieri A. Cardiovascular consequences of laparoscopic surgery. Lancet 1998;352:568-70. [Crossref] [PubMed]
- Rosenberg J, Fuchs-Buder T. Why surgeons need to know about anaesthesia. Surg Endosc 2016;30:3661-4. [Crossref] [PubMed]
- Atkinson TM, Giraud GD, Togioka BM, et al. Cardiovascular and Ventilatory Consequences of Laparoscopic Surgery. Circulation 2017;135:700-10. [Crossref] [PubMed]
- de Seigneux S, Klopfenstein CE, Iselin C, et al. The risk of acute kidney injury following laparoscopic surgery in a chronic kidney disease patient. NDT Plus 2011;4:339-41. [PubMed]
- Reijnders-Boerboom GTJA, Albers KI, Jacobs LMC, et al. Low intra-abdominal pressure in laparoscopic surgery: a systematic review and meta-analysis. Int J Surg 2023;109:1400-11. [Crossref] [PubMed]
- Fuchs-Buder T, Romero CS, Lewald H, et al. Peri-operative management of neuromuscular blockade: A guideline from the European Society of Anaesthesiology and Intensive Care. Eur J Anaesthesiol 2023;40:82-94. [Crossref] [PubMed]
- Laycock JR, Donati F, Smith CE, et al. Potency of atracurium and vecuronium at the diaphragm and the adductor pollicis muscle. Br J Anaesth 1988;61:286-91. [Crossref] [PubMed]
- Pansard JL, Chauvin M, Lebrault C, et al. Effect of an intubating dose of succinylcholine and atracurium on the diaphragm and the adductor pollicis muscle in humans. Anesthesiology 1987;67:326-30. [Crossref] [PubMed]
- Fernando PU, Viby-Mogensen J, Bonsu AK, et al. Relationship between posttetanic count and response to carinal stimulation during vecuronium-induced neuromuscular blockade. Acta Anaesthesiol Scand 1987;31:593-6. [Crossref] [PubMed]
- Lindekaer AL, Halvor Springborg H, Istre O. Deep neuromuscular blockade leads to a larger intraabdominal volume during laparoscopy. J Vis Exp 2013;50045. [PubMed]
- Pawlik MT, Prasser C, Zeman F, et al. Pronounced haemodynamic changes during and after robotic-assisted laparoscopic prostatectomy: a prospective observational study. BMJ Open 2020;10:e038045. [Crossref] [PubMed]
- Whiteley JR, Taylor J, Henry M, et al. Detection of elevated intracranial pressure in robot-assisted laparoscopic radical prostatectomy using ultrasonography of optic nerve sheath diameter. J Neurosurg Anesthesiol 2015;27:155-9. [Crossref] [PubMed]
- Katayama S, Mori K, Pradere B, et al. Influence of steep Trendelenburg position on postoperative complications: a systematic review and meta-analysis. J Robot Surg 2022;16:1233-47. [Crossref] [PubMed]
Cite this article as: Rosenberg J, Fuchs-Buder T. Low-pressure pneumoperitoneum—why and how. Laparosc Surg 2023;7:15.


