Laparoscopic hepatic resections for colorectal cancer metastases: a narrative review
Introduction
Development of laparoscopic liver resection (LLR)
Laparoscopic surgery has substantially transformed surgical practice during the last 30 years. The development in instrumentation and surgical skills gradually led to an ever-expanding list of surgical indications. Technological innovations involved also the hepato-pancreato-biliary (HPB) field, and the first LLRs for a benign liver lesion was reported in early 1990s.
From these years on, several cases were reported. In 1991, Reich et al. (1), describes their first experience in resecting superficial lesions of the liver edge found incidentally during laparoscopic surgery for gynecologic symptoms managed by a laparoscopic approach.
In 1992, Gagner et al. (2) reported a difficult laparoscopic liver surgery of a 6 cm focal nodular hyperplasia (FNH); a large segment IV resection was performed through ultrasonic dissector, monopolar cautery, and clip appliers.
Few years later, in 1995, Ferzli et al. (3) reported the laparoscopic resection of a voluminous hepatic adenoma, by the use of ultrasonic dissector and endoscopic vascular staplers.
The improvement in surgical experience and the introduction of new endoscopic devices allowed the laparoscopic approach of a greater number of intra-abdominal organs, and some procedures considered in the past exclusively performable in open technique are now attempted laparoscopically with increasing success.
An important contribution to LLR comes from Azagra et al. (4) in 1996: he successful performed a pure laparoscopic left lateral segmentectomy in a woman with a symptomatic benign adenoma of segments II and III.
Compared to other field of abdominal surgery, the laparoscopic surgery in liver resection has not been rapidly developed because it was considered technically demanding.
In addition, the role of laparoscopic surgery for liver malignancies did not find an immediate consensus for oncological reasons: some concerns were raised against the safety of resection margins, the risk of disease dissemination (seeding and squeezing), and the risk of misdiagnose small metastases.
Among the first series on LLR, we find Hüscher et al., in 1998 (5), with their first 38 liver resections, including right and right extended hepatectomy. This series is important because, for the first time, most cases were malignant diseases [15 hepatocellular carcinoma (HCC) and 17 liver metastasis] and out of the 29 patients that were followed-up, 17 patients were alive and free of disease, 10 developed recurrent disease (and 3 of whom died), 1 patient developed a different cancer and 1 patient showed signs of liver cirrhosis.
This showed that LLR was finally feasible and it could be considered safe also in case of malignancy.
An important prospective evaluate on by Cherqui et al. (6) in 2000, demonstrated the technical feasibility of not extended LLRs in very selected patients with small lesions. No detectable port-site or intraperitoneal metastases was reported, and there wasn’t any case of early tumor recurrences. Still from Paris, Brice Gayet published in 2004 one of the first larger case series on laparoscopic liver surgery including major anatomical resections for CRLM (7).
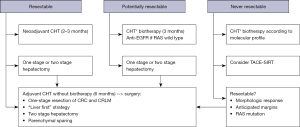
In November 2008, the Louisville Consensus Conference stated several recommendations, in particular the consensus about safety of laparoscopic approach; minimal-invasive surgery could be considered effective in the management of surgical liver disease in selected cases, when performed by trained surgeons with experience in both hepatobiliary and laparoscopic surgery (Figure 1).

According to Louisville consensus, the indications to laparoscopic resections were: solitary lesions, smaller than 5 cm, situated in liver segments from 2 to 6. This means that a left lateral sectionectomy can be approached by laparoscopy as standard practice (8).
At the opposite, a certain skepticism remained through the major resections. In 2014, the second international consensus conference on LLR finally clarify the world position on laparoscopic liver surgery and the interest through this technique increased rapidly. Initially described for benign lesions, mostly peripheral, eligible for wedge resections, LLRs became more frequent, even for larger, malignant tumors located in “unfavorable” segments. In a landmark review of LLRs in 2009 Nguyen et al. (9) sorted through 2,804 minimally invasive liver resections stating that, in experienced hands, the laparoscopic approach is safe also in case major hepatic resections. Furthermore, the oncological results at 3- and 5-year reported for HCC and colorectal cancer (CRC) metastases were comparable to open hepatic resection.
In recent years, mostly after the Second Consensus Conference that was held on 2014, in Morioka (10), with the validation for the safe development and progression of laparoscopic liver surgery the trend was reversed providing evidence to the superiority of LLR over open surgery in some short-term outcomes for both HCC and CRLM. Another important key point of the 2nd consensus was the concept of “parenchyma sparing” anatomical resection.
Later described by Kazaryan et al. (11), multiple concomitant liver resections provide surgical and oncologic outcomes comparable with single greater resections for multiple lesions.
Objectives
In this review, we aim to describe the current role of LLR in colorectal metastases and discuss its future perspectives. We present this article in accordance with the Narrative Review reporting checklist (available at https://ls.amegroups.com/article/view/10.21037/ls-22-42/rc).
Methods
Scopus, PubMed and Cochrane databases were searched. The search was limited to the English articles, published from January 1990 to June 2022 and for which full text was available (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | June 2022 to November 2022 |
| Databases and other sources searched | PubMed, Scopus, Cochrane |
| Search terms used | Colorectal liver metastasis, liver resection, hepatectomy, minimally invasive, laparoscopic liver resection, liver surgery, augmented reality, image-guided surgery, artificial intelligence, indocyanine green, 3D reconstructions, robotic liver surgery |
| Timeframe | 1990–2022 |
| Inclusion and exclusion criteria | Inclusion: full text articles and reviews in English. Colorectal liver metastasis |
| Exclusion: pediatric population, case reports | |
| Selection process | The articles identified were screened by the first author based on abstracts and relevant articles were selected. The selected articles were reviewed and their reference lists screened for further relevant literature outside the initial search time line |
The following keywords were used to search in titles or abstracts: “colorectal liver metastasis”, “liver resection”, “hepatectomy”, “minimally invasive”, “laparoscopic liver resection”, “liver surgery”, “augmented reality”, “image-guided surgery”, “artificial intelligence”, “Indocyanine green”, “3D reconstructions”, “robotic liver surgery”.
Our enquiry was restricted to original articles, cases series and reviews.
Treatment strategy for colorectal liver metastases (CRLM)
CRC remains a leading cause of tumor-related morbidity and mortality worldwide (12); it is ranked as third in terms of incidence (10.2% of all cancer cases worldwide) and is considered the second most common cause of cancer mortality (9.2% of all cancer mortality) in the world. However, tremendous improvements were reached in term of survival in patients with CRC (13) when considering that the reported 2-year overall survival (OS) for stage IV CRC was only 21% in the 1990s (14). In the last 2 decades, the 5-year OS progressively increased from 9.1% to 19.2%, mostly thanks to an increase in the patients eligible to hepatic resection and to the improvement in systemic chemotherapy (15).
The hematogenous spreading via the portal circulation of cancer cells from CRC makes the liver an easy target for metastatic dissemination. The presence of liver metastasis represents the most critical prognostic factor considering that the reported incidence of synchronous metastases is 15–25% (16) and up to 18–25% patients will develop distant metachronous metastases within 5 years from the first diagnosis (17).
A strong prognostic role is played by primary tumor biology. Concerning CRC, by now, KRAS and BRAF are the best know mutations. It is known that tumors with KRAS mutation are associated with worst prognosis due to adverse response to targeted anti-EGFR therapy as well as to oxaliplatin or irinotecan-based chemotherapy; on the clinical point of view, this sub group of patients have lower chance of presenting with resectable liver metastases and higher risk of extra hepatic disease. The poorer survival has been found in association of RAS mutation imposes an accurate selection of the patient before proposing aggressive surgical therapies (18).
Multidisciplinary approach, combining systemic chemotherapy, the administration of biologic agents and surgical resection permit to improve survival even for stage IV patients previously eligible only to palliative treatment.
From 1999 starting from the Clinical Risk Score from Fong et al. (19), many “score systems” have been created to try to better select patients and correlate pre-operative data with prognosis and long-term survival. Unfortunately, their role remains really limited; decision for surgery in patients with CRLM is a complex task and many factors must be considered.
Stage IV CRC encloses a wide clinical spectrum of disease, but overall, the median survival ranges from 5 to 20 months in absence of treatment. Among these, in approximately 20% to 30% of patients the disease is confined to the liver, allowing a surgical approach. Thanks to the improvement of surgical strategies adopted in many hepatobiliary centers, hepatectomy of even 70% of the liver can be performed, with a mortality rate below 5% and a 6-year survival of nearly 40% (20).
Concerning CRLM laparoscopic surgical management was previously considered a contraindication due to inability to achieve wide tumor-free resection margins (21).
Nowadays, liver resection for CRLM has become the gold standard treatment and provides the best chance of long-term outcomes, with an OS rate of 40% (22).
All these encouraging data justify the optimism regarding the increasingly aggressive approach being offered to many of patients with liver metastases from CRC.
What we surely know is that complete surgical resection of liver metastases represents the only potentially curative treatment for patients with CRLM.
Minimally invasive surgery and loco-regional treatment in CRLM
Beside surgery, there is a large spectrum of interventional radiology procedures which are considered as either valid alternatives to surgery or complementary treatments, including percutaneous ablation [radiofrequency (RFA), microwaves (MWA)], trans-arterial chemoembolization (TACE), and selective internal radiation therapy (SIRT).
These treatments are indicated in selected patients presenting CRLM not eligible to surgery (including patient decision) and which can be treated with a curative intent.
Talking about loco regional treatments, Solbiati et al. (23) reported 3-, 5- and 10-year survival rates of 69%, 48% and 18%, respectively in a series of 202 CRLM treated with RFA; RFA was also considered the major complication rate was 1.3% and there were no procedure-related deaths.
As an alternative to RF, MWA has progressively gained attention, presenting even some advantages compared with RFA ablation, such us increased infra-tumor temperature, shorter ablation time, and greater ablation range, thus being less affected by the vascular heat sink effect (24). According to Mimmo et al. (25), global OS rates at 3, 6 months, and 1-, 3-, and 5-year were 99.3%, 97.3%, 86.7%, 59.6%, and 44.8%, respectively.
Talking about locoregional treatments and surgery, the currently ongoing randomized phase III COLLISON trial (26) is trying to compare the results of thermal ablation in both open and laparoscopic resection in case of CRLM, with the purpose to provide more definitive conclusions.
Concerning patients affected by CRC oligometastatic disease in whom a second line chemotherapy failed or patients not suitable for surgery or other loco-regional treatments intra-arterial therapies can be an option.
TACE can be performed using chemotherapy drugs associated with either emulsions composed by ethiodized oil (conventional TACE) or drug-eluting beads (DEB-TACE); in the case of a trans-arterial radioembolization (TARE) the most common agent used is the Yttrium-90.
Both strategies aim to obtain a local tumor control and an OS prolongation (27).
Concerning the role of radioembolization, SIRFLOX, a randomized phase III study, showed that the addition of SIRT-selective internal radiation therapy, to FOLFOX based chemotherapy in patients with liver-dominant or liver-only metastatic CRC significantly delayed the interval of disease progression in the liver. In some cases, this permit conversion of patients with unresectable HCC to surgical candidates (28).
Different scenarios can be present and surgeons and oncologists face frequent concomitant liver and colorectal disease. This means that multidisciplinary strategies can be proposed:
- In case of both easy primary tumor and liver resection: synchronous resection.
- In case of easy primary tumor resection but borderline or unresectable liver tumors: neoadjuvant chemotherapy, followed by liver resection and finally primary tumor resection.
- In case of difficult or unresectable primary tumor resection but easy liver resection: neoadjuvant chemotherapy for primary tumor (or chemo-radiotherapy for rectal lesions), followed by primary tumor resection and finally hepatectomy.
One-stage resection of colorectal cancer and liver metastases
With improvement of perioperative management and neoadjuvant chemotherapy resection of primitive cancer and liver metastases is now considered safe.
The advantages of a synchronous approach include a single surgical procedure under general anesthesia, shorter cumulative length of hospital stay with associated reduction in resource utilization and health care costs. Most data on synchronous resections includes patients with limited hepatic disease demanding for limited liver resections.
Kleive et al. (29) concluded that simultaneous resection should be restricted to selected patients with a limited liver tumor burden.
Several unresolved issues concerning simultaneous resections still exist: first, no randomized trial has been realized; furthermore, the ideal number of metastases that can be considered safe to resect is not established, so that patient selection can be challenging.
Additionally, Driedger et al. (30) demonstrated the negative effect of postoperative complications on long-term outcomes especially in case of simultaneous resection concerning the delay or even failure to receive planned postoperative chemotherapy, concluding that this approach should be avoided.
“Liver first” strategy
The “liver-first” approach or “reverse” strategy provides the resection of the liver metastases as the first step in the management of synchronous disease, including the administration of preoperative chemotherapy and the resection of CRLM followed by the resection of the primary tumor at a second stage.
First described by Mentha et al. (31), the liver first strategy showed better results in term of resectability and survival, with 86% of the patients completing the total program of treatments; moreover, these patients showed 3- and 5-year survival of 60% and 31%.
In patients with multiple bi-lobar liver metastases, liver-first approach gives priority to the treatment of the most prognostically relevant tumor. This theoretically means a better OS over both the primary-first and the simultaneous approaches. Three-years survival rates after reverse strategy exceeded 65% and ranged between 50 and 60% in the other groups according to Giuliante et al. (32).
In addition, the liver first strategy allows to take full advantages of a multidisciplinary approach because hepatectomies can be performed with optimal timing after the neoadjuvant chemotherapy, without any treatment interruption and at the optimal peak of the morphologic and biologic response. All this was also possible thanks to the parallel development of CRC management, especially in the field of chemo-radiotherapy that can lead to complete endoscopic, radiological and clinical response, letting to avoid pelvic surgery or colonic stenting that permits to manage partial obstruction without resorting to urgent bowel surgery.
While planning this strategy, limiting the duration of preoperative chemotherapy to less than 6 cycles and ensuring adequate future liver remnant volume (FLRV) and an appropriate liver volume to body weight ratio (LVBWR) before surgery will be vital for a successful colorectal metastases resection.
A further argument in favor of the reverse strategy is that in the primary-first approach should always consider that colorectal surgery (but especially the postoperative complication potentially arising from these surgical procedures) could expose to immunosuppression, favoring the spreading of metastatic proliferation. Patients with post operative complications had a significant reduction in OS and disease-free survival (DFS) (33).
Two stage hepatectomy (TSH)
Only the 15–25% of patients with CRLM have resectable liver disease at the diagnosis and extensive bi-lobar liver disease remain a therapeutic challenge for both oncologists and surgeons. The main issue is the need to preserve an adequate disease-free FLRV to prevent post-hepatectomy liver failure (PHLF) due to a small-for-size syndrome.
The accepted FLRV changes according to liver status: for patients with no liver diseases, without any signs of cirrhosis, or those who never underwent chemotherapy, the optimal volume of the future liver remnant is estimated at least 20–25% in case of prior chemotherapy a minimum FLRV of 30% is required before resection (34).
In this setting, Adam et al. (35) first proposed in 2000 the TSH strategy: this technique consists in combining two sequential liver resection when it is impossible to resect all liver metastases in a single procedure.
With the purpose to maximize the liver’s regenerative potential enhancing the hypertrophy of the FLR, right portal vein ligation (RPVL) performed during the first step of a TSH, or the subsequent radiological right portal vein embolization (RPVE) were introduced.
Encouraging results come from Narita et al. (36) who reported their first 10 years of experience concluding that TSH is a therapeutic strategy that provides acceptable long-term survival with no postoperative mortality.
In 2003, Jaeck et al. (37) described a one- or two-stage hepatectomy combined with portal vein embolization (PVE) for initially non-resectable CRLM assessing feasibility and interesting outcomes in patients with initially unresectable CRLM. Moreover, 3-year survival was similar to that observed in patients with initially resectable liver metastases.
Levi Sandri et al. (38) presented in 2015 their 10 years’ experience with TSH: 80% of patients could complete the surgery; for patients who couldn’t undergo the second stage procedure, the reported prognosis was almost the same than those treated with chemotherapy alone. In the same series, a totally laparoscopic first-stage operation was performed in 5 patients.
Moreover, there were zero complications rate during the first stage and during the second stage all the well-known advantages of laparoscopic surgery were evident, such us less adhesions and shorter lengths of hospital stay compared to open approaches. This means, above all, faster patients’ recovery and a short interruption of the chemotherapy regimen (39).
In experienced hands, the second stage hepatectomy can be performed completely laparoscopically. An accurate patient’s selection is recommended, considering that a laparoscopic approach in these cases might be challenging because of a technically demanding hilar dissection as a consequence of PV embolization or previous ligation, with a complication rate up to 59% (40).
Total laparoscopic TSH was compared to open technique by Okumura et al. (41) in a bi-institutional propensity score-matched study, showing that total laparoscopic approach is safe and feasible in selected patients. Less blood loss and less post operative complications were reported in the laparoscopic group, as well as a shorter hospital stay. This translated into a faster recovery, allowing the patients belonging to the laparoscopic TSH group to receive adjuvant chemotherapy 2 weeks earlier.
Moreover, laparoscopic TSH showed a 3- and 5-year OS comparable to open approach. These results suggest that the laparoscopic approach has no negative effect on oncological outcomes in patients treated with TSH for bi-lobar CRLM (42).
However, a diagnosis of bi-lobar CRLM remain a challenge for liver surgeons, as classical anatomic major resections may compromise the amount of liver remnant.
Two major strategies are possible within TSH:
- PVE;
- Associating Liver Partition and Portal Vein Ligation for Staged hepatectomy (ALPPS).
PVE (Figure 2): is still considered the gold standard for inducing liver hypertrophy. It was initially performed by surgery it can nowadays can be done by percutaneous puncture injecting embolic materials in ipsilateral or contralateral Portal Vein. It can induce slowly hypertrophy with a risk of tumor progression (43).
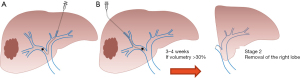
ALPPS (Figure 3): firstly introduced in 2012, associating liver partition and portal vein ligation for staged hepatectomy (ALPPS), let remove extensive part of the liver in two steps: in the first one a in situ splitting along the intended line of resection is performed. At the same time, the future liver remnant is “cleaned” by partial resections from all tumor tissue. A portal ligation of the larger liver lobe that will have to be removed is added. After a waiting period of at least 10 days the second step is performed and the previously “deportalised” liver is removed.

ALPPS is more appropriate in the settings of CRLM than in primary liver and biliary malignancies. It induces a rapid liver hypertrophy increasing the resection rate with a slightly higher mortality and morbidity. It can also be used as a “rescue treatment” in case of PVE failure (44).
Parenchymal sparing hepatectomy (PSH)
Behind the different multi-step approaches, a turning point in treatment of bi-lobar CRLM was the PSH approach, first introduced by Gold et al. (45) in 2008.
In contrast with anatomic resection, whose surgical principle is based on the complete removal of an anatomical area defined by the vascular supply of the Glissonian branches, PSH is a de-escalation strategy with the aim of resecting only metastasis; this reduces the risk of stimulating tumor growth and allows iterative interventions. Furthermore, sparing healthy liver parenchyma increases its tolerance to chemotherapy.
When compared to upfront surgery, in particularly major hepatectomies, PSH showed lower 90-day mortality, less postoperative complications, less blood transfusions and shorter in-hospital stay (45).
PSH was initially performed only by open approach, until the OSLO-COMET trial clearly reported less postoperative complications and same rate of free margins, highlighting advantages deriving from a laparoscopic approach (46).
Kazaryan et al. (11) demonstrated a superiority of multiple parenchyma-sparing concomitant liver resections over early widely performed single major resection for multiple CRLM.
This approach could be recommended for a wide application in specialized hepatopancreatobiliary centers.
PSH resections is now possible and safe also in posterosuperior segments, always considered as unfavorable for a minimally invasive approach. Using a few tricks, such as changing intraoperatively the patient position (semiprone or left lateral position) or introducing transthoracic ports, also the more challenging posterosuperior segments resections became feasible when performed by trained surgeons (47).
With a PSH approach the sacrifice of liver parenchyma is minimum; indeed, the margin width seems narrow still permitting a R0 resection, with a clearance of bi-lobar multiple CRLM by one-stage resection of parenchymal-sparing intent provided comparable long-term survival to uni-lobar disease (48).
D’Hondt et al. (49) reported a 5-years OS of 76% for bi-lobar CLRM versus 66% for uni-lobar CRLM (P=0.49) treated through one stage laparoscopic parenchymal sparing liver resection, showing no significant difference in survival rates between bi-lobar and uni-lobar metastasis. Moreover, no significant differences in recurrence-free survival (RFS) at 1-, 3- and 5-year were reported the two groups (1-year 64% vs. 73%; 3-year 38% vs. 42%, 5-year 38% vs. 28%, P=0.62).
All these results support the feasibility and the safety of a laparoscopic parenchymal sparing liver resection for bi-lobar CRLM.
A multidisciplinary assessment is, however, paramount for an adequate patient selection and optimization and for better assess surgical timing within a multimodal approach (Figure 4).
Discussion
Liver surgery is likely to be performed in specialized centers and has traditionally been associated with a long learning curve and relatively high complication rates. This can explain why laparoscopic liver surgery has been slowly implemented.
The initial lack of evidence concerning oncological outcomes also contributed to the delayed development of this approach. Data on safety, feasibility, and oncological equivalence of the laparoscopic approach versus open surgery for CRLM resection is the key point.
According to a recent review from Taillieu et al. (50), there was no paper reporting negative conclusion on the minimal invasive approach in CRLM: good short- and long-term outcomes can be achieved independent of the complexity of the procedure.
A complete analysis by Tian et al. (51) comparing laparoscopic and open liver resection for CRLM showed no significant difference between laparoscopic and open resection in terms of operative time, while the intra-operative blood loss was significantly lower in LLR than in open liver resection and the proportion of patients requiring blood transfusion was lower in LLR than in open liver resection. This means that minimal invasive surgery can provide not only the same oncological outcomes but better surgical results.
This can be due to the increased abdominal pressure due to pneumoperitoneum and to the magnification of the images, that also means a better and more precise dissections of the vasculo biliary structures.
Moreover, LLR is performed by small incisions while open surgery often needs a bilateral subcostal or J-shaped incisions; in the last case the muscular and parietal damage increase the risk of postoperative pulmonary complications. Smaller incisions also mean less post-operative pain and earlier rehabilitation.
Concerning postoperative mortality and morbidity there are no differences between laparoscopic and open surgery and the highest reported 90-day postoperative mortality for LLR in general was 2.3% (52).
Laparoscopic liver surgery can also provide an economic benefit, reducing hospital stay and morbidity.
Concerning the quality of resection, Topal et al. (53) reported a median tumor-free resection margin of 7.5 mm in the laparoscopic group versus 5.5 mm in the open group (P=0.651). In this series, the median DFS of the entire study population was 18.4 months, the median OS was 50.7 months, demonstrating estimated DFS and OS rates at 1-, 2-, and 5-year comparable in the two groups (P=0.637 and 0.872, respectively).
The real interest of LLR in patients with CRLM would be the potential superiority on the oncological point of view: decreasing postoperative complications can have at least two advantages: an “oncologic” effect due to a reduction in inflammatory response and a decrease in recurrence (54); on the other side the time interval between LLR for CRLM and adjuvant chemotherapy is shorter compared to open surgery (43±10 versus 55±18 days, P=0.012) (55).
Technical consideration
LLR can furtherly be divided into: “pure” laparoscopy, hand-assisted laparoscopy (HALLR), and “hybrid technique”.
“Pure” laparoscopy means that the resection is entirely completed by laparoscopic ports.
When there is an elective placement of a hand port during LLR, it is better to talk about HALLR. The “hybrid technique” starts as a pure laparoscopy but the resection is performed through a mini-laparotomy incision. This is the so called “laparoscopy-assisted” technique.
In particular HALLR has been mostly used for peripheral and small lesions, and it offers the advantages of rapid bleeding control but the real advantages on open surgery still have to be proved (56).
In case of difficulties during pure laparoscopic resection, conversion to HALS instead that to open laparotomy can be a valid alternative.
The Hybrid technique can be useful in case of intra-abdominal adhesions or when a multiple partial hepatectomy (for e.g., bilobular multiple liver tumors) must be performed (57).
In order to overcome some conventional limits of LLR, the introduction of 3D (Figure 5) visualization led to a better visualization, enhancing the image magnification and improving surgical dissection. Furthermore, 3D visualization may reduce the operating time compared to high-definition 2D (58).
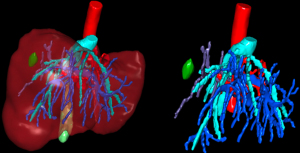
Further advances in technologies led to the application of indocyanine green ICG thanks to the development of NIR cameras ICG has an hepatic clearance and, once injected before surgery, enable to better visualize liver anatomy in and intrahepatic lesions, especially when well differentiated. When administered intraoperatively, ICG let identify the portal territories and guide more precise anatomical liver resections. Moreover, ICG technology has a diagnostic role in identification of new lesions and permit the characterization of known hepatic lesions in real time during liver resection (59).
For liver surgeons, anatomy knowledge is crucial. In order to facilitate complex surgical procedure, the use of augmented reality (AR) technology can be another useful tool. AR enable better and more accurate blood vessels and tumor structures visualization, allowing for precise navigation during complicated surgical procedures. It has been proved to be safe and effective. By now a lot of different (3D) image-processing software are available and they can provide a more accurate preoperative volumetric analysis for a safer surgery. This kind of tool can also allow a preoperative simulation of the planned resection in order to reduce the risk of unexpected liver necrosis (60).
Since 2000, robotic surgery utilization is increasing worldwide. The robotic system can be really useful in liver surgery because it combines the advantages of laparoscopic technique adding the dexterity and ergonomics. In addition, the robotic surgery perfectly fits with new technology such as AR, that can overcome the limit of tactile feedback and space orientation of surgeons. AR-based intraoperative reconstructions and tracking systems help surgeons to localize tumors and improve surgical results with well-defined preoperative planning or increased intraoperative detection (61).
By now, it remains to be proved if robotic surgery really give an advantage over standard laparoscopy in liver surgery.
On one side, operative time is longer with the robotic platform compared to standard laparoscopy. On the other side, robotic surgery seems to prove a real advantage in minor resections of the posterior segments.
Conclusions
Laparoscopic liver surgery has been progressively integrated into the panel of strategies treatment for CRLM.
At the beginning, the major limitation was the safety of surgical interventions with the purpose of having the lower complication rate. Furthermore, the fear of inferior oncological outcomes was also a great limitation.
By now, there are a large number of studies in favor of LLR.
It’s a matter of fact that laparoscopic livery surgery offers undoubtful advantages compared to open surgery. CRLM have been demonstrated to be suitable for laparoscopic approaches.
There are several retrospective studies that confirms the many advantages of LLR for CLRM, such as less blood loss and transfusion rate, better post operative pain control, less pulmonary complications. This means earlier hospital discharge and less hospital stay with an important impact on costs.
LLR for CRLM showed surgical superiority compared to open surgery and, after initial skepticism, many series showed no difference in oncological results with 3- and 5-year survival rates comparable to open hepatic resection, in selected group of patients.
In 2018 the first randomized controlled trial (RCT) from Fretland et al. (46), the OSLO COMET Trial, showed that laparoscopic technique significantly reduce the frequency of complications in liver resection for CRLM. The OSLO COMET showed significantly reduction in blood loss, lower operative transfusion requirement, shorter hospital stay, reduced overall morbidity, and reduced severe morbidity compared with conventional open surgery.
Long term oncological results are still on going.
Some limitations of LLR in CRLM can be represented by giant lesions that can be technically demanding. Anyway, when feasible, well-known advantages of minimally invasive surgery have also been confirmed also in this case.
For multilobar liver metastases minimal invasive surgery has a controversial role: it’s necessary to perfectly manage the principals of parenchymal sparing dissection and liver ultrasound.
More than for anatomical resections, 3D laparoscopy, ICG and 3D reconstruction with virtual reality can help to overcomes the difficulties on multiple atypical liver resections and parenchymal dissection.
LLR in case of CRLM in posterosuperior segments and biliary reconstructions still remain challenging.
In particular, Filmann et al. (62), showed that the need for a biliodigestive anastomosis increased mortality to 25.5 per cent.
Robotic liver surgery can possibly overcome these limitations.
This narrative review tries to give an overall vision of the history of laparoscopic surgery for CRLM, the state of art, and the potential limitations that should be overcome. The major limitations are linked to the design of the paper. Based on the current paper, we suggest LLR should be a standard procedure for colorectal metastases in selected patients. Some limitations of laparoscopic liver surgery, above all in case of biliary or vascular reconstruction, could be overcome by robotic surgery.
Further large-scale prospective studies are warranted to validate our findings.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Roberto Santambrogio and Marco Antonio Zappa) for the series “Laparoscopic Hepato-Biliary Surgery” published in Laparoscopic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://ls.amegroups.com/article/view/10.21037/ls-22-42/rc
Peer Review File: Available at https://ls.amegroups.com/article/view/10.21037/ls-22-42/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ls.amegroups.com/article/view/10.21037/ls-22-42/coif). The series “Laparoscopic Hepato-Biliary Surgery” was commissioned by the editorial office without any funding or sponsorship. GBLS serves as the Editor-in-Chief of Laparoscopic Surgery. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript in ensuring that questions related to the accuracy or integrity of any part of the manuscript are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reich H, McGlynn F, DeCaprio J, et al. Laparoscopic excision of benign liver lesions. Obstet Gynecol 1991;78:956-8. [PubMed]
- Gagner M, Rheault M, Dubuc J. Laparoscopic partial hepatectomy for liver tumor. Surg Endosc 1992;6:99.
- Ferzli G, David A, Kiel T. Laparoscopic resection of a large hepatic tumor. Surg Endosc 1995;9:733-5. [Crossref] [PubMed]
- Azagra JS, Goergen M, Gilbart E, et al. Laparoscopic anatomical (hepatic) left lateral segmentectomy-technical aspects. Surg Endosc 1996;10:758-61. [Crossref] [PubMed]
- Hüscher CG, Lirici MM, Chiodini S. Laparoscopic liver resections. Semin Laparosc Surg 1998;5:204-10. [PubMed]
- Cherqui D, Husson E, Hammoud R, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg 2000;232:753-62. [Crossref] [PubMed]
- Vibert E, Perniceni T, Levard H, et al. Laparoscopic liver resection. Br J Surg 2006;93:67-72. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg 2009;250:831-41. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Kazaryan AM, Aghayan DL, Barkhatov LI, et al. Laparoscopic Multiple Parenchyma-sparing Concomitant Liver Resections for Colorectal Liver Metastases. Surg Laparosc Endosc Percutan Tech 2019;29:187-93. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Fitzmaurice C, Abate D, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2019;5:1749-68. [Crossref] [PubMed]
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009;27:3677-83. [Crossref] [PubMed]
- van der Geest LGM, Lemmens VEPP, de Hingh IHJT, et al. Nationwide outcomes in patients undergoing surgical exploration without resection for pancreatic cancer. Br J Surg 2017;104:1568-77. [Crossref] [PubMed]
- van Gestel YR, de Hingh IH, van Herk-Sukel MP, et al. Patterns of metachronous metastases after curative treatment of colorectal cancer. Cancer Epidemiol 2014;38:448-54. [Crossref] [PubMed]
- Tosi F, Magni E, Amatu A, et al. Effect of KRAS and BRAF Mutations on Survival of Metastatic Colorectal Cancer After Liver Resection: A Systematic Review and Meta-Analysis. Clin Colorectal Cancer 2017;16:e153-e163. [Crossref] [PubMed]
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21. [Crossref] [PubMed]
- Valderrama-Treviño AI, Barrera-Mera B, Ceballos-Villalva JC, et al. Hepatic Metastasis from Colorectal Cancer. Euroasian J Hepatogastroenterol 2017;7:166-75. [Crossref] [PubMed]
- Shirabe K, Takenaka K, Gion T, et al. Analysis of prognostic risk factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin. Br J Surg 1997;84:1077-80. [PubMed]
- Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002;235:759-66. [Crossref] [PubMed]
- Solbiati L, Ahmed M, Cova L, et al. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology 2012;265:958-68. [Crossref] [PubMed]
- Facciorusso A, Serviddio G, Muscatiello N. Local ablative treatments for hepatocellular carcinoma: An updated review. World J Gastrointest Pharmacol Ther 2016;7:477-89. [Crossref] [PubMed]
- Mimmo A, Pegoraro F, Rhaiem R, et al. Microwave Ablation for Colorectal Liver Metastases: A Systematic Review and Pooled Oncological Analyses. Cancers (Basel) 2022;14:1305. [Crossref] [PubMed]
- Puijk RS, Ruarus AH, Vroomen LGPH, et al. Colorectal liver metastases: surgery versus thermal ablation (COLLISION) - a phase III single-blind prospective randomized controlled trial. BMC Cancer 2018;18:821. [Crossref] [PubMed]
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386-422. [Crossref] [PubMed]
- van Hazel GA, Heinemann V, Sharma NK, et al. SIRFLOX: Randomized Phase III Trial Comparing First-Line mFOLFOX6 (Plus or Minus Bevacizumab) Versus mFOLFOX6 (Plus or Minus Bevacizumab) Plus Selective Internal Radiation Therapy in Patients With Metastatic Colorectal Cancer. J Clin Oncol 2016;34:1723-31. [Crossref] [PubMed]
- Kleive D, Aas E, Angelsen JH, et al. Simultaneous Resection of Primary Colorectal Cancer and Synchronous Liver Metastases: Contemporary Practice, Evidence and Knowledge Gaps. Oncol Ther 2021;9:111-20. [Crossref] [PubMed]
- Driedger MR, Yamashita TS, Starlinger P, et al. Synchronous resection of colorectal cancer primary and liver metastases: an outcomes analysis. HPB (Oxford) 2021;23:1277-84. [Crossref] [PubMed]
- Mentha G, Roth AD, Terraz S, et al. 'Liver first' approach in the treatment of colorectal cancer with synchronous liver metastases. Dig Surg 2008;25:430-5. [Crossref] [PubMed]
- Giuliante F, Viganò L, De Rose AM, et al. Liver-First Approach for Synchronous Colorectal Metastases: Analysis of 7360 Patients from the LiverMetSurvey Registry. Ann Surg Oncol 2021;28:8198-208. [Crossref] [PubMed]
- Dorcaratto D, Mazzinari G, Fernandez M, et al. Impact of Postoperative Complications on Survival and Recurrence After Resection of Colorectal Liver Metastases: Systematic Review and Meta-analysis. Ann Surg 2019;270:1018-27. [Crossref] [PubMed]
- Gruttadauria S, Vasta F, Minervini MI, et al. Significance of the effective remnant liver volume in major hepatectomies. Am Surg 2005;71:235-40. [Crossref] [PubMed]
- Adam R, Laurent A, Azoulay D, et al. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg 2000;232:777-85. [Crossref] [PubMed]
- Narita M, Oussoultzoglou E, Jaeck D, et al. Two-stage hepatectomy for multiple bilobar colorectal liver metastases. Br J Surg 2011;98:1463-75. [Crossref] [PubMed]
- Jaeck D, Bachellier P, Nakano H, et al. One or two-stage hepatectomy combined with portal vein embolization for initially nonresectable colorectal liver metastases. Am J Surg 2003;185:221-9. [Crossref] [PubMed]
- Levi Sandri GB, Santoro R, et al. Two-stage hepatectomy, a 10 years experience. Updates Surg 2015;67:401-5. [Crossref] [PubMed]
- Levi Sandri GB, Colace L, Vennarecci G, et al. Laparoscopic first step approach in the two stage hepatectomy. Hepatobiliary Surg Nutr 2015;4:345-7. [PubMed]
- Di Fabio F, Whistance R, Rahman S, et al. Exploring the role of laparoscopic surgery in two-stage hepatectomy for bilobar colorectal liver metastases. J Laparoendosc Adv Surg Tech A 2012;22:647-50. [Crossref] [PubMed]
- Okumura S, Goumard C, Gayet B, et al. Laparoscopic versus open two-stage hepatectomy for bilobar colorectal liver metastases: A bi-institutional, propensity score-matched study. Surgery 2019;166:959-66. [Crossref] [PubMed]
- Fuks D, Nomi T, Ogiso S, et al. Laparoscopic two-stage hepatectomy for bilobar colorectal liver metastases. Br J Surg 2015;102:1684-90. [Crossref] [PubMed]
- Del Basso C, Gaillard M, Lainas P, et al. Current strategies to induce liver remnant hypertrophy before major liver resection. World J Hepatol 2021;13:1629-41. [Crossref] [PubMed]
- Sparrelid E, Hasselgren K, Røsok BI, et al. How should liver hypertrophy be stimulated? A comparison of upfront associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) and portal vein embolization (PVE) with rescue possibility. Hepatobiliary Surg Nutr 2021;10:1-8. [Crossref] [PubMed]
- Gold JS, Are C, Kornprat P, et al. Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome: trends in treatment over time in 440 patients. Ann Surg 2008;247:109-17. [Crossref] [PubMed]
- Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, et al. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg 2018;267:199-207. [Crossref] [PubMed]
- Okumura S, Tabchouri N, Leung U, et al. Laparoscopic Parenchymal-Sparing Hepatectomy for Multiple Colorectal Liver Metastases Improves Outcomes and Salvageability: A Propensity Score-Matched Analysis. Ann Surg Oncol 2019;26:4576-86. [Crossref] [PubMed]
- Fukami Y, Kaneoka Y, Maeda A, et al. Bilobar versus unilobar multiple colorectal liver metastases: a propensity score analysis of surgical outcomes and recurrence patterns. J Hepatobiliary Pancreat Sci 2017;24:153-60. [Crossref] [PubMed]
- D'Hondt M, Pironet Z, Parmentier I, et al. One-stage laparoscopic parenchymal sparing liver resection for bilobar colorectal liver metastases: safety, recurrence patterns and oncologic outcomes. Surg Endosc 2022;36:1018-26. [Crossref] [PubMed]
- Taillieu E, De Meyere C, Nuytens F, et al. Laparoscopic liver resection for colorectal liver metastases - short- and long-term outcomes: A systematic review. World J Gastrointest Oncol 2021;13:732-57. [Crossref] [PubMed]
- Tian ZQ, Su XF, Lin ZY, et al. Meta-analysis of laparoscopic versus open liver resection for colorectal liver metastases. Oncotarget 2016;7:84544-55. [Crossref] [PubMed]
- Allard MA, Cunha AS, Gayet B, et al. Early and Long-term Oncological Outcomes After Laparoscopic Resection for Colorectal Liver Metastases: A Propensity Score-based Analysis. Ann Surg 2015;262:794-802. [Crossref] [PubMed]
- Topal H, Tiek J, Aerts R, et al. Outcome of laparoscopic major liver resection for colorectal metastases. Surg Endosc 2012;26:2451-5. [Crossref] [PubMed]
- Fretland AA, Sokolov A, Postriganova N, et al. Inflammatory Response After Laparoscopic Versus Open Resection of Colorectal Liver Metastases: Data From the Oslo-CoMet Trial. Medicine (Baltimore) 2015;94:e1786. Correction appears in Medicine (Baltimore) 2016;95:e367e.
- Kawai T, Goumard C, Jeune F, et al. Laparoscopic liver resection for colorectal liver metastasis patients allows patients to start adjuvant chemotherapy without delay: a propensity score analysis. Surg Endosc 2018;32:3273-81. [Crossref] [PubMed]
- Lin S, Wu F, Wang L, et al. Surgical outcomes of hand-assisted laparoscopic liver resection vs. open liver resection: A retrospective propensity score-matched cohort study. Chin J Cancer Res 2019;31:818-24. [Crossref] [PubMed]
- Hasegawa Y, Koffron AJ, Buell JF, et al. Approaches to laparoscopic liver resection: a meta-analysis of the role of hand-assisted laparoscopic surgery and the hybrid technique. J Hepatobiliary Pancreat Sci 2015;22:335-41. [Crossref] [PubMed]
- Velayutham V, Fuks D, Nomi T, et al. 3D visualization reduces operating time when compared to high-definition 2D in laparoscopic liver resection: a case-matched study. Surg Endosc 2016;30:147-53. [Crossref] [PubMed]
- Lim C, Vibert E, Azoulay D, et al. Indocyanine green fluorescence imaging in the surgical management of liver cancers: current facts and future implications. J Visc Surg 2014;151:117-24. [Crossref] [PubMed]
- Berardi G, Colasanti M, Meniconi RL, et al. The Applications of 3D Imaging and Indocyanine Green Dye Fluorescence in Laparoscopic Liver Surgery. Diagnostics (Basel) 2021;11:2169. [Crossref] [PubMed]
- Giannone F, Felli E, Cherkaoui Z, et al. Augmented Reality and Image-Guided Robotic Liver Surgery. Cancers (Basel) 2021;13:6268. [Crossref] [PubMed]
- Filmann N, Walter D, Schadde E, et al. Mortality after liver surgery in Germany. Br J Surg 2019;106:1523-9. [Crossref] [PubMed]
Cite this article as: Del Basso C, Usai S, Levi Sandri GB. Laparoscopic hepatic resections for colorectal cancer metastases: a narrative review. Laparosc Surg 2023;7:13.