
Strategy and technique of pancreatojejunostomy in minimally invasive pancreaticoduodenectomy: clinical practice review
Introduction
Pancreaticoduodenectomy (PD) for periampulary tumors is a highly difficult surgery, and postoperative complications such as pancreatic fistula (PF) are frequent (1) (n=8,575). Under such circumstances, laparoscopic surgery for PD was reported in 1994 (2) (n=1), and robot-assisted surgery has gradually spread since the report from Giulianotti et al. in 2003 (3) (n=193). When laparoscopic or robot-assisted surgery are proposed, it is important to ensure the equivalent curability and safety of open surgery. From this point of view, it is very important to review the papers published so far on pancreaticojejunum (PJ) anastomosis in laparoscopic or robotic PD. Therefore, in this article, we will review the past literature and also show our own results of laparoscopic or robotic PD.
Technique of laparoscopic PJ anastomosis
It is not difficult to imagine that laparoscopic PJ anastomosis would be quite difficult, but there are several papers that have explored how to safely perform laparoscopic anastomosis, and some of these are introduced in this section.
Many surgeons may employ the Blumgart procedure for PJ anastomosis in open surgery (4), and Poves et al. provide a detailed and illustrated explanation of the laparoscopic method (5) (n=13). They state that when a PJ mucosa anastomosis is conducted, two threads that have penetrated the pancreatic parenchyma in advance (usually one each in the cephalocaudal direction and not crossing the main pancreatic duct) can be led out of the body through one trocar to maintain proper alignment with the PJ mucosa anastomosis. While performing a Blumgart anastomosis, it is often difficult to ligate the pancreatic parenchyma and jejunal wall without loosening sutures. Nagakawa et al. reported a simple suture method to solve such a situation, using LAPRA-TY (6) (n=19). The frequency of clinical PF was not different when compared with the conventional Blumgart procedure (n=20), however, the time for PJ anastomosis was significantly reduced.
When the main pancreatic duct diameter is very small or cannot be identified, performing a laparoscopic pancreatic jejunal anastomosis is not straightforward. Cho et al. reported a useful method of dunking in such cases (7) (n=15). The method itself does not seem to be difficult, so we recommend that you refer to the video clip of the article, in which a thin stent was inserted into the main pancreatic duct. The preliminary results of a randomized controlled trial on the usefulness of stent in PJ anastomosis in laparoscopic pancreaticoduodenectomy (LPD) were reported recently by Cai et al (8), and concluded that there was no difference in anastomosis time or incidence of PF with (n=49) or without (n=41) stent. PJ anastomoses in LPD are easier when done with a stent, and in this randomized controlled trial, even in the no-stent group, the stent was inserted once in the handling of the needle and removed before ligation when anastomosis was performed, with the authors recommending this procedure even in the non-stent maneuver.
We consider that the difficulty of laparoscopic PJ anastomosis lies largely in the linear design of the forceps. In a paper with a video, Bibo et al. introduced a PJ anastomosis technique using an articulating needle-holder (9). At first glance, it could be mistaken for robot-assisted surgery, and the impression is that it is clearly easier to suture than a regular needle-holder. Further studies are required to assess this technique, its effectiveness, and its potential.
Some facilities may perform pancreaticogastrostomy (PG) anastomosis as a routine procedure, or as an alternative to PJ anastomosis when the main pancreatic duct is not visible. Matsuda et al. reported a laparoscopic PG anastomosis without pancreatic ductal mucosa anastomosis with a video (10) (n=5). The anterior wall was also incised, and pancreatogastric anastomosis was performed in the gastric cavity. Although there are few data on the long-term outcome of this procedure, if it can be performed easily and safely, it is an alternative method that should be mastered. Conversely, some institutions may hesitate to perform LPD, due to the difficulty to perform the PJ anastomosis, even if the resection can be performed laparoscopically. In such cases, laparoscopic resection followed by reconstruction under direct vision, in which a small laparotomy is made on the ventral side of the planned PJ anastomosis, may be indicated. We have used this method extensively in the era of laparoscopic resection, with satisfactory results in terms of postoperative course (11) (n=20). Whether this procedure should be called laparoscopic-assisted surgery or hybrid surgery is still unclear. If there is a strong commitment to perform the reconstruction under the direct vision, this method may be useful even in the current era of laparoscopic surgery.
Technique of robotic PJ anastomosis
Unlike laparoscopic PJ anastomosis, there are few papers dedicated to robotic PJ anastomosis technique. This may be because the articulated function allows robotic PJ anastomosis to be performed in the same way as in open surgery and does not require much special ingenuity comparing to LPD. The approach to robotic PJ anastomosis can be roughly divided into two groups: one is to suture the pancreatic parenchyma with continuous sutures, and the other is to suture with Blumgart anastomosis or minor modifications resembling open abdominal surgery. As for continuous suture, Liu et al. reported a detailed method, naming it single-layer continuous anastomosis (12). The pancreatic stent is inserted as an internal stent and fixed with 5-0 suture. The results were also excellent, reporting a pancreatic anastomosis time of 14.9 minutes and a grade B PF frequency of 6.7% in continuous suture anastomosis group (n=89). On the other hand, Takagi et al. in their educational article on robotic pancreaticoduodenectomy (RPD) describe in detail the PJ anastomosis method using the interrupted two-layer modified Blumgart method they have learned in the Netherlands (13). The distinctive feature of this method is the ligation and fixation of the posterior wall before the PJ mucosa anastomosis. We have also performed PJ anastomosis using a similar method, which we describe in detail here.
First, a straight needle, non-absorbable suture is used to penetrate the pancreatic parenchyma from the ventral side to the dorsal side, and then the intestinal wall is penetrated coaxially with the intestinal canal to form a u-shape. Normally, three stitches are made, one around the main pancreatic duct and one each in the cephalocaudal direction. Depending on the size of the pancreatic parenchyma, only two stitches may be made (Figure 1). These are ligated from the cranial side thread first, since the posterior wall is fixed in advance. It is important that the jejunal wall is properly laid on the dorsal surface of the pancreatic parenchyma and ligated. Since there is no sense of touch in robotic surgery, it is necessary to appropriately ligate based on the appearance of the closing of the pancreatic parenchyma (Figure 2). Next, a small hole is made in the jejunum, and the pancreatic jejunal mucosa is anastomosed using 5-0 monofilament absorbable thread with 6 stitches every 60 degrees. The first stitch is placed at the 7 o’clock position, viewed from the jejunal side, and the thread is deployed and secured with a No. 4 arm forceps to maintain alignment of the small hole in the jejunum with the main pancreatic duct (Figure 3). After completing 4 stitches every 60 degrees of the posterior wall of the main pancreatic duct, we always insert a 10 cm 5-Fr internal-stent before anastomosis of the anterior wall with 2 remaining stitches. Although there is no data confirming usefulness of internal-stent in preventing PF (14) (n=45), we think that it is useful to perform anterior wall stitches (Figure 4). After suturing the main pancreatic duct with a total of 6 stitches, the anterior wall of the jejunum and the anterior wall of the pancreatic parenchyma are sutured with a straight needle thread that has previously been used to fix the posterior wall of the parenchyma. Since this ligation is easily loosened due to tension, the thread on the caudal side is pre-tightened with arm No. 4 to prevent the cranial side ligation from loosening (Figure 5).
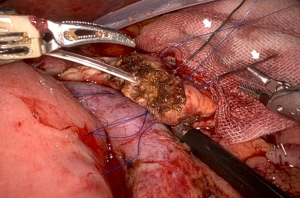
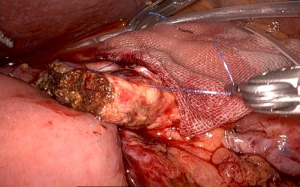
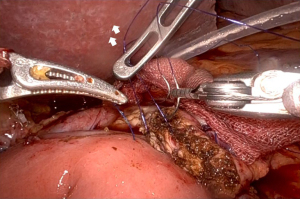

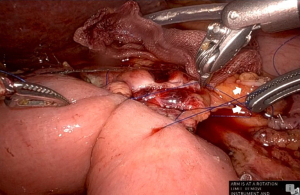
Comparison of the results between laparoscopic and robotic PD
We have presented methods for laparoscopic and robot-assisted PJ anastomosis. Several papers have already compared the results of these two approaches, which will be presented in this section. In a retrospective study using NSQIP data (280 laparoscopic cases and 211 robotic cases), Zimmerman et al. reported the following LPD vs. RPD data: operative time [421 vs. 404 minutes, not significant (ns.)], conversion rate to open (28% vs. 11.4%, P<0.001), PF incidence (19.2% vs. 21.9%, ns.), overall morbidity (55% vs. 62.1%, ns.), mortality rate (2.5% vs. 2.8%, ns.); similar results for both techniques, except for conversion rate (15). Regarding conversion to laparotomy, Lof et al. analyzed the results of a European multicenter retrospective study [overall: 457 laparoscopic and 250 robotic; conversion to laparotomy: 52 laparoscopic (11.3%) and 13 robotic (5.2%)] and found that laparoscopic surgery was associated with a higher rate of conversion to open surgery. The authors also compared the results of patients who underwent laparotomy: operative time (conversion from LPD vs. RPD: 370 vs. 480 min, P=0.004), PF incidence (25% vs. 15%, ns.), and mortality rate (6% vs. 23%, ns.). The high mortality rate in converted RPDs is alarming, but they cited only one bleeding event as the reason for conversion from RPDs, with tumor vascular invasion being the main reason for the rest (16). In a paper published this year from Korea, the authors compared LPD and RPD cases with a propensity score match, from 280 LPD and 80 RPD cases to 74 LPD and 74 RPD cases, and found that the operation time (LPD vs. RPD: 452 vs. 411 min, P=0.001), the conversion rate (8.1% vs. 0%, P<0.001), PF rate (13.5 vs. 12.2%, ns.), complication beyond Clavien-Dindo classification IIIa (14.9% vs. 21.6%, ns.), length of hospital stay (14.6 vs. 11.9 days, P=0.027), and concluded that RPD has advantages over LPD in several respects (17). In addition to the articles above, our own experience (91 cases of LPD and 28 cases of RPD) were shown in Table 1. LPDs were performed by two surgeons including first author and all RPDs were performed by a single surgeon (TA) in our department. The indication for LPD or RPD for periampullary tumor is the same at our institution, and excluding the borderline resectable pancreatic cancer, those requiring vascular reconstruction and bile duct cancer located with hilar side from the cystic duct in each procedure. The results at our institution show that, although the operative time is still long, blood loss, PF, and complication rates are acceptable for both LPD and RPD, and the RPD clearly outperforms the LPD especially in the aspect of surgical safety. Our results for RPD may further improve with more experience because the technical skills of the surgeons should improve. For this reason, we believe that RPD is preferable to LPD.
Table 1
| Variables | LPD (n=91) | RPD (n=30) | P |
|---|---|---|---|
| Operative time (min) | 535 [373–995] | 557 [387–875] | 0.49 |
| Blood loss (mL) | 250 [10–2,550] | 49 [20–200] | <0.001 |
| PF | 14 (15%) | 1 (3%) | 0.08 |
| CD3a ≤ | 21 (23%) | 2 (7%) | 0.04 |
| Hospital stay (days) | 23 [9–109] | 18 [7–78] | 0.04 |
Data are presented as median [range] or n (%). LPD, laparoscopic pancreaticoduodenectomy; RPD, robotic pancreaticduodenectomy; PF, clinically relevant pancreas fistula; CD3a, complication beyond Clavien-Dindo 3a.
Conclusions
In summary, this article summarizes the technical methods and comparative results of LPD and RPD, especially in PJ anastomosis. The results of the PJ anastomosis in RPD were seemed not inferior to those of the LPD in the past literature at least with respect to safety, and were superior than that of LPD in the results of our department. However, randomised controlled trial (RCT) is needed to clarify the true comparison between the results of LPD and RPD.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ippei Matsumoto) for the series “Laparoscopic Pancreatic Surgery” published in Laparoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ls.amegroups.com/article/view/10.21037/ls-22-57/coif). The series “Laparoscopic Pancreatic Surgery” was commissioned by the editorial office without any funding or sponsorship. SE serves as an unpaid editorial board member of Laparoscopic Surgery from September 2022 to August 2024. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kimura W, Miyata H, Gotoh M, et al. A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: the 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg 2014;259:773-80. [Crossref] [PubMed]
- Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408-10. [Crossref] [PubMed]
- Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003;138:777-84. [Crossref] [PubMed]
- Blumgart LH, Fong Y. editors. Pancreaticojejunostomy. Surgery of the liver and biliary tract, 3rd ed. Philadelphia: Saunders; 2000:1073-89.
- Poves I, Morató O, Burdío F, et al. Laparoscopic-adapted Blumgart pancreaticojejunostomy in laparoscopic pancreaticoduodenectomy. Surg Endosc 2017;31:2837-45. [Crossref] [PubMed]
- Nagakawa Y, Takishita C, Hijikata Y, et al. Blumgart method using LAPRA-TY clips facilitates pancreaticojejunostomy in laparoscopic pancreaticoduodenectomy. Medicine (Baltimore) 2020;99:e19474. [Crossref] [PubMed]
- Cho A, Yamamoto H, Kainuma O, et al. Performing simple and safe dunking pancreaticojejunostomy using mattress sutures in pure laparoscopic pancreaticoduodenectomy. Surg Endosc 2014;28:315-8. [Crossref] [PubMed]
- Cai H, Lu F, Zhang M, et al. Pancreaticojejunostomy without pancreatic duct stent after laparoscopic pancreatoduodenectomy: preliminary outcomes from a prospective randomized controlled trial. Surg Endosc 2022;36:3629-36. [Crossref] [PubMed]
- Bibo L, Ballal M. Laparoscopic Pancreaticojejunostomy Anastamosis Using an Articulating Needle Holder. Surg Laparosc Endosc Percutan Tech 2021;32:279-80. [Crossref] [PubMed]
- Matsuda M, Haruta S, Shinohara H, et al. Pancreaticogastrostomy in pure laparoscopic pancreaticoduodenectomy--A novel pancreatic-gastric anastomosis technique. BMC Surg 2015;15:80. [Crossref] [PubMed]
- Kuroki T, Adachi T, Okamoto T, et al. A non-randomized comparative study of laparoscopy-assisted pancreaticoduodenectomy and open pancreaticoduodenectomy. Hepatogastroenterology 2012;59:570-3. [Crossref] [PubMed]
- Liu Q, Zhao Z, Gao Y, et al. Novel Technique for Single-Layer Pancreatojejunostomy is Not Inferior to Modified Blumgart Anastomosis in Robotic Pancreatoduodenectomy: Results of a Randomized Controlled Trial. Ann Surg Oncol 2021;28:2346-55. [Crossref] [PubMed]
- Takagi K, Umeda Y, Yoshida R, et al. Surgical training model and safe implementation of robotic pancreatoduodenectomy in Japan: a technical note. World J Surg Oncol 2021;19:55. [Crossref] [PubMed]
- Kuroki T, Tajima Y, Kitasato A, et al. Stenting versus non-stenting in pancreaticojejunostomy: a prospective study limited to a normal pancreas without fibrosis sorted by using dynamic MRI. Pancreas 2011;40:25-9. [Crossref] [PubMed]
- Zimmerman AM, Roye DG, Charpentier KP. A comparison of outcomes between open, laparoscopic and robotic pancreaticoduodenectomy. HPB (Oxford) 2018;20:364-9. [Crossref] [PubMed]
- Lof S, Vissers FL, Klompmaker S, et al. Risk of conversion to open surgery during robotic and laparoscopic pancreatoduodenectomy and effect on outcomes: international propensity score-matched comparison study. Br J Surg 2021;108:80-7. [Crossref] [PubMed]
- Kim H, Choi SH, Jang JY, et al. Multicenter comparison of totally laparoscopic and totally robotic pancreaticoduodenectomy: Propensity score and learning curve-matching analyses. J Hepatobiliary Pancreat Sci 2022;29:311-21. [Crossref] [PubMed]
Cite this article as: Adachi T, Matsushima H, Imamura H, Hara T, Soyama A, Hidaka M, Kanetaka K, Eguchi S. Strategy and technique of pancreatojejunostomy in minimally invasive pancreaticoduodenectomy: clinical practice review. Laparosc Surg 2023;7:3.


