
Laparoscopic resection of hepatic hilum schwannoma: a case report focusing on reliable imaging criteria and minimally invasive approach feasibility
Introduction
A schwannoma is a benign tumor arising from Schwann cells of the nerve sheath (1). Schwannomas occur at all ages, but tumor incidence is higher between 20 and 50 years and are less common in paediatric age (2).
Spinal roots, cervical sympathetic nerves, vagus, peroneal and ulnar nerves are the most common site where schwannomas develop and therefore it can be usually observed on head, neck and extremities flexor surfaces (3). The gastrointestinal tract and the retroperitoneal cavity are considered a rare localization of this kind of lesion.
Primary benign schwannoma of the porta hepatis is almost unique (4). Hence, we report a case of 62 years old woman affected by porta hepatis schwannoma treated with curative laparoscopic resection. We present the following case in accordance with the CARE reporting checklist (available at https://ls.amegroups.com/article/view/10.21037/ls-22-14/rc).
Case presentation
A Caucasian 62-year-old woman was referred to our hepato-pancreato-biliary unit in December 2021 for an incidental finding of a perihepatic lesion at abdominal ultrasound.
She had no comorbidities with the exception of hypothyroidism on medication nor previous surgery but a family history of gastric cancer.
Therefore, a positron emission tomography/computed tomography (PET/CT) showed a 50 mm × 35 mm oval mass of uncertain origin in the hepatic hilum, with evidence of pathological enhancement [standardized uptake value (SUV) max 3.5]. In axial arterial phase the hypodense lesion was located al hepatic hilum and surrounded by noble vascular structures: medially by celiac trunk and common hepatic artery, laterally main portal trunk and posteriorly inferior vena cava. An expert radiologist suspected this lesion to be a schwannoma since having these two peculiar features: a mesenchymal-like CT pattern as being hysointense to muscles in every sequence and a posterior tail in continuity with the presumptive hilum nerves location. No other lesions were revealed within the liver or other distant sites (Figure 1).
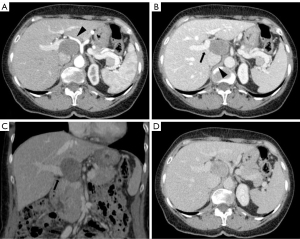
After multidisciplinary team (MDT) discussion of the case, surgical removal of the lesion was indicated for presumptive diagnosis of schwannoma. The patient was scheduled for elective laparoscopic resection. Operative position was supine. Peritoneal access was gained through para-rectal incision and Hasson’s trocar placement, followed by insertion of four operative trocars. Exploratory laparoscopy did not highlight any lesion of other abdominal organs and confirmed the presence of an oval mass between hepatoduodenal ligament folds, in an inter-porto-caval position. Pringle maneuver was performed. Surgery proceeded with hepatic hilum’s structures preparation and left gastric, common hepatic, proper hepatic and hepatic hilum stations lymphadenectomy (7, 8a-p, 12a-b-p). Accurate dissection of lax adhesions between the neoplasia and the hilar elements and the inferior vena cava followed. Specimen extraction with Endobag through pararectal access. Operating time was 2 hours 45 minutes. She started to drink clear liquids within 5 hours and was mobilized. On 1 postoperative day (POD) she was already in good condition, ate and drank without any problem and bowel was already opened to flatus and stool. Laboratory tests were unremarkable and she was discharged on the second postoperative day.
Histological examination revealed a yellow mass (4.5 cm × 4 cm) with macroscopic necrotic areas when cut. Microscopic examination revealed a capsulated lesion with areas of hypercellularity of spindle cells without atypia (Antoni A pattern). Immunohistochemistry stained positive for vimentin, S-100 and SOX10, typical neuroendocrine tissue markers. Expression of p16 was conserved and maximal mitotic activity was 4 per 10 high power field. There was no vascular infiltration nor metastatic lesions in examined lymph-nodes. Overall, this picture was compatible with a cellular Schwannoma [World Health Organization (WHO) 2020] with R0 resection margins (Figure 2).
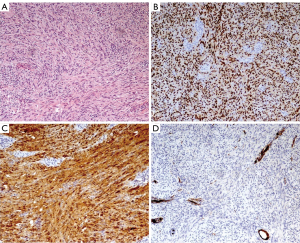
Due to lesion benignity, no further treatment was indicated after MDT discussion. The patient is alive in good clinical condition at 1-year follow-up.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Schwannoma is a benign tumour that originates from Schwann cells of the nerve sheath (5). Schwannoma usually develops along peripheral nerves, the dorsal spinal nerve roots or cranial nerves, while primary benign schwannoma of the liver is absolutely rare (6). The hepatobiliary nerves originate from the hepatic plexus in the hilum. A schwannoma of the liver can originate from a variety of hepatic sympathetic and parasympathetic nerves distributed among the intralobular connective tissues and hepatic arteries (7). The most frequent type of schwannoma is the sporadic one although it can be associated with neurofibromatosis 2 or 1, with schwannomatosis or with multiple meningiomas (8). Malignant transformation of these tumors is infrequent.
Typical schwannoma features are slow growth, a well-defined capsule and they are usually smaller than 5 cm at diagnosis (9). It’s possible that larger schwannomas undergo secondary degeneration such as haemorrhage, calcification or pseudocystic regression, which sometimes leads to misdiagnosis of degenerated hydatid cyst on CT (10,11). Generally schwannoma looks as a non-homogenous area on plain CT, and show clear margins and an irregular internal pattern on enhanced CT (12). Magnetic resonance (MR) can be employed as second line imaging technique; at MR the evidence of isointense signal to muscle both on T1 and on T2 images, can suggest mesenchymal origin of the lesion excluding other potential tumors. After gadolinium administration several patterns have been described, with the most common being hypervascular behavior; furthermore, in presence of secondary degeneration, the signal can be different and not specific. Moreover, in some cases the neural origin can be also depicted (13). Even 18F-fluorodeoxyglucose (F-FDG) PET/CT difficulty solve the diagnostic doubt (14). In fact a presumptive diagnosis of primary hepatic schwannoma by radiological methods was possible in our case but remains difficult and a definitive one is usually possible just with histological analysis (14,15).
Histologically conventional schwannomas are usually an encapsulated spindle cell tumor that is composed nearly entirely of well-differentiated Schwann cells as many others mesenchymal tumors. The differences between a schwannoma and other stromal tumor are the presence of a capsule and the contemporary existence of two different patterns named Antoni A and Antoni B. The Antoni A area is dense and cellular, while the Antoni B is hypocellular. Immunohistochemical staining shows a diffuse and strong positivity for S-100 protein in cell nuclei and cytoplasm; similarly, SOX10 immunoreactivity is usually extensive. Expression of glial fibrillary acidic protein (GFAP) is less frequent and more variable (5).
The differential diagnosis between schwannoma and other spindle cell tumors is mandatory. First of all, we excluded gastrointestinal stromal tumor due to C-kit and DOG-1 negativity. Furthermore, negative staining for smooth muscle differentiative markers excluded the diagnosis of leiomyoma (7,16). Finally, among peripheral nerve sheet tumors, the negative stain for CD34 excluded the diagnosis of neurofibroma. In fact, while schwannomas are usually benign, neurofibromas show a more complex histological and clinical features (17).
The treatment for benign primary schwannoma is complete excision (7). Complete excision is curative without the need of additional treatment after surgery (18). Postoperative course is usually uncomplicated and the overall prognosis is very good. There are two other cases reported in the literature: one was a hepatoduodenal ligament schwannoma treated with open surgery excision, the other one in retroperitoneal space between common hepatic artery and left gastric artery excised laparoscopically (19,20). In both cases mass excision was curative and no further treatment was deemed necessary.
In our case laparoscopic approach provided an optimal view of the mass which was easily excised. For the above reason we suggest that laparoscopic approach is feasible, safe and allows an en bloc mass resection. A great advantage of laparoscopic surgery is that since it is less invasive, we were able to discharge our patient on 2 POD, which would have been very difficult in patient undergoing open surgery. Limitation of this study is that, since is a unique case report, its reproducibility needs to be verified by study with major evidence such as a case series or a systematic review of literature.
Conclusions
Primary benign schwannomas of hepatic hilum are extremely rare. There are typical radiological features that could lead to a presumptive diagnosis but a preoperative diagnosis of certainty remains challenging, therefore histological analysis is essential. Complete excision is curative and in experienced centres it could be achieved through a laparoscopic approach which appears safe, feasible and guarantees faster recovery.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://ls.amegroups.com/article/view/10.21037/ls-22-14/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ls.amegroups.com/article/view/10.21037/ls-22-14/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity and the originality of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mankin HJ, Mankin KP. Schwannoma: a rare benign tumor of soft tissues. Musculoskelet Surg 2014;98:95-9. [Crossref] [PubMed]
- Voss BL, Pysher TJ, Humphrey GB. Peripheral neuroepithelioma in childhood. Cancer 1984;54:3059-64. [Crossref] [PubMed]
- Knight DM, Birch R, Pringle J. Benign solitary schwannomas: a review of 234 cases. J Bone Joint Surg Br 2007;89:382-7. [Crossref] [PubMed]
- Kapoor S, Tevatia MS, Dattagupta S, et al. Primary hepatic nerve sheath tumor. Liver Int 2005;25:458-9. [Crossref] [PubMed]
- Wanebo JE, Malik JM, VandenBerg SR, et al. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 28 cases. Cancer 1993;71:1247-53. [Crossref] [PubMed]
- Wada Y, Jimi A, Nakashima O, et al. Schwannoma of the liver: report of two surgical cases. Pathol Int 1998;48:611-7. [Crossref] [PubMed]
- Lee WH, Kim TH, You SS, et al. Benign schwannoma of the liver: a case report. J Korean Med Sci 2008;23:727-30. [Crossref] [PubMed]
- Hoda SA. Enzinger and Weiss’s Soft Tissue Tumors. Am J Clin Pathol 2020;154:424. [Crossref]
- Demetri GD, Baker LH, Beech D, et al. Soft tissue sarcoma clinical practice guidelines in oncology. J Natl Compr Canc Netw 2005;3:158-94. [PubMed]
- Cohen LM, Schwartz AM, Rockoff SD. Benign schwannomas: pathologic basis for CT inhomogeneities. AJR Am J Roentgenol 1986;147:141-3. [Crossref] [PubMed]
- Yoshida A, Yamao K, Takenaka M, et al. Neurilemmoma Mimicking a Multilocular Cystic Lesion of the Liver. Intern Med 2018;57:3377-80. [Crossref] [PubMed]
- Picchia S, Terlizzo M, Bali MA. Hepatic schwannoma: CT and histologic features. Curr Probl Cancer 2019;43:511-3. [Crossref] [PubMed]
- Lee NJ, Hruban RH, Fishman EK. Abdominal schwannomas: review of imaging findings and pathology. Abdom Radiol (NY) 2017;42:1864-70. [Crossref] [PubMed]
- Liu Y, Xu B. Hepatic Schwannoma on 18F-FDG PET/CT. Clin Nucl Med 2020;45:808-10. [Crossref] [PubMed]
- Rossi P, Sileri P, Gentileschi P, et al. Percutaneous liver biopsy using an ultrasound-guided subcostal route. Dig Dis Sci 2001;46:128-32. [Crossref] [PubMed]
- Daimaru Y, Kido H, Hashimoto H, et al. Benign schwannoma of the gastrointestinal tract: a clinicopathologic and immunohistochemical study. Hum Pathol 1988;19:257-64. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 2006;130:1466-78. [Crossref] [PubMed]
- Ozkan EE, Guldur ME, Uzunkoy A. A case report of benign schwannoma of the liver. Intern Med 2010;49:1533-6. [Crossref] [PubMed]
- Heffron TG, Coventry S, Bedendo F, et al. Resection of primary schwannoma of the liver not associated with neurofibromatosis. Arch Surg 1993;128:1396-8. [Crossref] [PubMed]
- Nishizawa N, Kumamoto Y, Hirata M, et al. Retroperitoneal schwannoma between the inferior vena cava and the abdominal aorta resected by laparoscopic surgery: A case report. Asian J Endosc Surg 2015;8:361-4. [Crossref] [PubMed]
Cite this article as: Anselmo A, Siragusa L, Sensi B, Silvestri F, Petagna L, Proietti G, Ferlosio A, Argirò R, Tisone G. Laparoscopic resection of hepatic hilum schwannoma: a case report focusing on reliable imaging criteria and minimally invasive approach feasibility. Laparosc Surg 2022;6:30.


