Factors influencing recurrence after minimally invasive treatment of hiatal hernia—a single center experience
Introduction
Since 1991, when Dallemagne demonstrated the possibility to apply minimally invasive surgery to anti-reflux procedure (1), laparoscopic fundoplication has become the standard technique (2). The debate of laparoscopic vs. open surgery is no longer ongoing because of the clear decline of surgery-related morbidity and mortality after the introduction of the laparoscopic approach (3). The application of robotic in this field was the natural technical evolution as in other surgical procedures, in order to improve surgical performance by eliminating tremors and fatigue and allowing articulation of the instrument a three-dimensional view. Different approaches have been proposed to repair hiatal hernias: reduction, sac excision, gastropexy, posterior cruroplasty (4-6), mesh reinforcement of posterior cruroplasty (6-8), and several types of fundoplication (9,10).
Anyway, recurrent intrathoracic migration has been reported to have a relatively high rate (1,11). A majority of recurrences is believed to be asymptomatic (1,12-14). Recurrence that needs reintervention occurs more uncommonly, with an estimated 10-year cumulative risk of approximatively 7% (15), however, reoperations have a significantly higher morbidity and mortality than their primary counterparts. It is likely that the risk of recurrence is multifactorial, and many different factors that could affect the durability of hernia repair have been previously proposed (16).
The aim of this study was to focus on factors that may be linked to a higher risk of recurrence after minimally invasive Nissen fundoplication for hiatal hernia to allow a better preoperative selection of the patients in order to maximize the benefit of minimally invasive surgery.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/ls-20-63).
Methods
A database of patients undergoing minimally invasive Nissen fundoplication was retrospectively examined to assess perioperative and postoperative outcomes at Clinica Chirurgica in Ancona, between 2012 and 2018. The research was approved by the local ethical committee and the informed consent was obtained from all patients. All the patients underwent endoscopy, esophagogram, manometry and, possibly, 24 hours pH-metry before surgery.
Inclusion criteria consisted of a positive surgical history for reflux treated with minimally invasive procedures (laparoscopic Nissen fundoplication, LNF, or robotic Nissen fundoplication, RNF) and one year follow up at least. Patients undergone partial fundoplication, laparotomic operation or lost at follow up were excluded.
The primary endpoint of this study was to find clinical, anatomic, and technical factors that could influence the success rate of minimally invasive surgery for hiatal hernias.
Demographic data, including age, gender, pre-existing comorbidity, BMI and ASA score were recorded. The size of the hernia was not considered a contraindication to one or another procedure. All the funduplications from 2014 onward were carried out robotically.
Patients were divided into four categories according to their preoperative BMI and based on Centers for Disease Control and Prevention (CDC) classification: normal (18.5 to <25), overweight (25.0 to <30), obese Class 1 (30 to <35), and a combination of obese Class 2 (35 to <40) and Class 3 (≥40).
On the basis of the literature (17,18), hiatal hernia were classified into four types according to their characteristics (17). Type 1 hernias included the sliding hiatal hernia, characterized by migration of the gastroesophageal junction (GEJ) into the posterior mediastinum. Hernias with herniation of the gastric fundus into the mediastinum alongside the oesophagus, with the GEJ remaining in an intraabdominal position were classified as Type 2 (true paraesophageal). Type 3 hernias (mixed hernias) involved herniation of the stomach with the GEJ into the mediastinum. Hernias characterized by the intrathoracic migration of the stomach along with associated viscera were classified as Type 4.
Time to surgery (TS) was also recorded defined as the time between the first endoscopic diagnosis of hiatal hernia and the time when surgery was performed.
Operative times, intraoperative complications, post-operative complications and length of hospital stay were collected. Early complications were defined as complications occurring during the hospital stay or within 30 days from surgery. Recurrence needing reoperation was defined as recurrent intrathoracic herniation of the stomach diagnosed either endoscopically or by radiological imaging.
Follow-up evaluation was performed at 1, 3, 12, 24, and 36 months after surgery.
Operative technique
In order to avoid biases due to technical changes data from 2012, when a standardized technique for Nissen fundoplication was used for all patients, were analysed. All the procedure were performed by the same surgeon. The first step pf the procedure was the intra-abdominal reduction of the hernia content, followed by division of the short gastric vessels with a bipolar electrothermal energy device in both LNF and RNF (LigaSureTM, Medtronic, in LNF and EndoWrist One VesselSealer, Intuitive Surgical, in RNF).
Then a mediastinal dissection of the oesophagus was performed in order to gain additional length and to put the GEJ back to a subdiaphragmatic position. Sometimes, when the GEJ was not easily returned to an intrabdominal position, a further lengthening procedure, known as Collis gastroplasty, was required (4). Retroesophageal crural primary closure was then performed approximating the crura by mean of 3 to 5 interrupted nonabsorbable barbed sutures (3/0 V-LocTM, Medtronic).
In all cases, the Nissen fundoplication was performed by wrapping the stomach fundus 360 around the esophagus at the end of the hiatoplasty, respecting DeMeesters criteria (a floppy and short valve calibrated on a 16-mm probe) (19). However, the wrap was not sutured to the oesophagus or the diaphragm.
Statistical analysis
Qualitative data were analysed by mean of Fisher’s exact test. Students t-test was used to compare quantitative data. Quantitative data have been shown as median and interquartile range (IQR), qualitative data as absolute value and percentage into brackets. The statistical significance was set at a two-tailed P<0.05. In univariate analysis, seven variable parameters were considered: gender, age (dichotomized at 65 years), ASA score, BMI (dichotomized at 25 and 30 kg/m2), TS (dichotomized at 3 years), type of hernia, type of procedure. Results were expressed as Odds Ratios (OR) and 95% CI.
The variables potentially related to recurrence in the univariate analysis (P<0.200) were subsequently entered into a multivariate analysis. The statistical significance was assessed at a level of probability of 0.05.
A Kaplan–Meier failure function coupled with long-rank test for equality of survivor functions was used to further evaluate factors related to recurrence.
All the statistical analysis were performed using the Med Calc statistical software.
Results
Among the 50 patients undergone minimally invasive fundoplication between 2012 and 2018 there were 14 males [LNF =12 (33.3%), RNF =2 (14.3%)] and 36 females [LNF =24 (66.6%); RNF =12 (85.7%); P=0.294]. Patients did not differ for baseline demographic characteristics.
Although it could be noticed a difference in the size of the hernia between RNF and LNF this was not a selection criteria for one or another procedure.
The median age was 70.9 years (IQR =67.4–7) (LNF =71.4, IQR =65.4–72.1), RNF= 67.5, IQR =63.7–70.4; P=0.827). Most of the patients were classified as ASA score II (28 56%; P=0.780) and most of them were over-weighted (22, 41.2%; P=0.221) or affected by Class I obesity (10, 20%; P=0.246). Most of the hiatal hernia were classifiable as Type 3 (19, 38%; P=0.521) (Table 1).
Table 1
| Variables | Total | LNF (n=36) | RNF (n=14) | P |
|---|---|---|---|---|
| Age, median (±SD) | 70.9 (13.2) | 71.4 (±14.4) | 67.5 (±10.2) | 0.827 |
| Gender, n (%) | ||||
| Male | 14 (28.0) | 12 (33.3) | 2 (14.3) | 0.294 |
| Female | 36 (72.0) | 24 (66.6) | 12 (85.7) | 0.294 |
| BMI, n (%) | ||||
| Normal | 15 (30.0) | 9 (25.0) | 6 (42.8) | 0.301 |
| Overweight | 22 (44.0) | 17 (47.2) | 5 (35.7) | 0.221 |
| Obese | 13 (26.0) | 10 (27.8) | 3 (21.4) | 0.730 |
| Class 1 | 10 (20.0) | 9 (25.0) | 1 (7.1) | 0.246 |
| Class 2 | 3 (6.0) | 1 (2.8) | 2 (14.2) | 0.185 |
| Class 3 | 0 | 0 | 0 | / |
| ASA score, n (%) | ||||
| ASA I | 15 (30.0) | 9 (25.0) | 6 (42.8) | 0.304 |
| ASA II | 28 (56.0) | 21 (58.3) | 7 (50.0) | 0.780 |
| ASA III | 7 (14.0) | 6 (16.6) | 1 (7.1) | 0.656 |
| Hernia Type, n (%) | ||||
| Type 1 | 3 (6.0) | 0 | 3 (21.4) | 0.018 |
| Type 2 | 15 (30.0) | 8 (22.2) | 7 (50.0) | 0.085 |
| Type 3 | 19 (38.0) | 15 (41.7) | 4 (28.5) | 0.521 |
| Type 4 | 13 (26.0) | 13 (36.1) | 0 | 0.011 |
LNF=Laparoscopic Nissen Fundoplication; RNF= Robotic Nissen Fundoplication.
There were no differences between the two groups regarding operative times (101.4 mins, IQR =80–115 mins; P=0.125), post-operative complications (6, 12%; P=0.196), length of hospital stay (7.37 days, IQR=8–6 days; P=0.245) and 30 days readmission rate (3, 6%; P=0.544) (Table 2).
Table 2
| Variables | Total | LNF (n=36) | RNF (n=14) | P |
|---|---|---|---|---|
| OT, median, mins (±SD) | 101.4 (±23.9) | 105 (23.9) | 90 (22.4) | 0.125 |
| IC, n (%) | 0 | 0 | 0 | – |
| LOS, median, mins (±SD) | 7.37 (3.2) | 7 (3.2) | 7 (4.0) | – |
| PC, n (%) | 6 (12.0) | 4 (11.1) | 2 (14.3) | 0.196 |
| 30 day readmission, n (%) | 3 (6.0) | 3 (8.3) | 0 | 0.544 |
| Recurrence, n (%) | 9 (18.0) | 8 (22.2) | 1 (7.1) | 0.246 |
LNF, laparoscopic Nissen fundoplication; RNF, robotic Nissen fundoplication; OT, operative time; IC, intra-operative complications; LOS, length of hospital stay; PC, post-operative complications.
The post-operative complications were 2 left fluid pleural collections, treated conservatively, and one bilateral, that needed the insertion of a drain, and one case of persistent dysphagia in the laparoscopic group.
In the robotic group one patient complained about dysphagia, and in one patient a transmural necrosis of the fundus and body of the stomach requiring total gastrectomy was observed.
Three patients (8.3%) in the laparoscopic group were readmitted 2 for dysphagia (5.5%) and 1 (2.8%) for abdominal pain. None of the patients in the robotic group required readmission.
Dysphagia was treated conservatively. Median time to recurrence was 8.7±14.7 months. The recurrence rate was 18%.
Class I Obesity (OR =9, 95% CI, 1.792–45.188; P=0.007), Type 3 hernia (OR =7.35, 95% CI, 1.238–43.694; P=0.028) and TS >3 years (OR =18, 95% CI, 3.195–101.385; P<0.001) (Table 3). On multivariate analysis the type of hiatal hernia (P=0.032), obesity (P=0.012) and TS (P=0.003) statistically correlated with recurrence, but this did not seem to be influenced by surgical procedure (P=0.300) (Table 4).
Table 3
| Variables | OR | 95% CI | P | |
|---|---|---|---|---|
| Lower | Upper | |||
| Gender [M |
1.09 | 0.238 | 4.996 | 0.910 |
| Age >65 |
0.88 | 0.193 | 4.047 | 0.874 |
| ASA I | 0.51 | 0.096 | 2.799 | 0.426 |
| ASA II | 0.73 | 0.184 | 2.964 | 0.669 |
| ASA III | 3.85 | 0.703 | 21.153 | 0.131 |
| Normal [<25 kg/m2] | 0.58 | 0.107 | 3.163 | 0.532 |
| Overweight [>25 kg/m2, < 30 kg/m2] | 0.52 | 0.118 | 0.118 | 0.383 |
| Obese [>30 kg/m2] | 4.71 | 1.069 | 20.789 | 0.041* |
| Class I [>30 kg/m2, <35 kg/m2] | 9 | 1.792 | 45.188 | 0.007* |
| Type 1 | 0 | – | – | 0.231 |
| Type 2 | 0.20 | 0.023 | 1.797 | 0.094 |
| Type 3 | 7.35 | 1.238 | 43.694 | 0.028* |
| Type 4 | 0.65 | 0.120 | 3.600 | 0.630 |
| Time to surgery [> 3 years] | 18 | 3.195 | 101.385 | <0.001 |
| Surgical procedure | 4.33 | 0.495 | 37.929 | 0.125* |
Table 4
| OR | 95% CI | P | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Class I [>30 kg/m2, < 35 kg/m2] | 10.992 | 1.660 | 72.783 | 0.012* |
| Type 2 | 1.12 | 0.062 | 20.118 | 0.857 |
| Type 3 | 7.36 | 0.800 | 67.698 | 0.032* |
| Time to surgery [>3 years] | 9.84 | 2.012 | 80.597 | 0.003* |
| Surgical procedure | 3.93 | 0.295 | 52.398 | 0.300 |
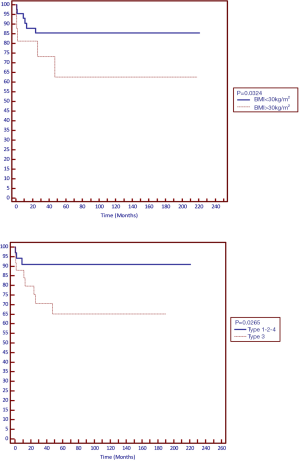
Further analysis of recurrence using Kaplan–Meier failure function and log-rank test for equality of survivors by surgical procedure showed significant difference in recurrence for patients with Class I obesity (P=0.034) and type of hiatal hernia (P=0.026) (Figure 1).
Two patients, one in each group needed had a short esophagus needing a Collis gastroplasty.
Discussion
In our series BMI and time between the first diagnosis and surgical intervention were found as factors related with a higher risk of recurrence of hiatal hernia after surgery.
Fundoplication is the standard surgical procedure to treat GERD. Previous studies have demonstrated that laparoscopic fundoplication should be chosen over the open counterpart, because although ensuring similar efficacy, it lowers morbidity and mortality rates (0.04% vs. 0.2%), with better cosmetic results (20-23).
The laparoscopic approach allows to achieve a better visualization of the hiatal region than that obtained with open surgery and this technical advantage is combined with lower morbidity and mortality rates, shorter hospital stay, and, therefore, better patient compliance. In addition, during the step of hernia reduction and dissection, with this approach it is possible to have a fine identification of surgical plans and anatomical structures, and the pneumoperitoneum itself may facilitate the dissection (4).
As in other field, robotic have been introduced in anti-reflux surgery with the aim to overcome the intrinsic technical challenges of laparoscopy. Its feasibility have been largely demonstrated (24), and, in our series, no differences were observed between LNF and RFN in terms of intraoperative and post-operative outcomes. Even operative times, that are often blamed to be one of the main disadvantages of robotic surgery, did not statistically differ and seemed to show the tendency to be even shorter in RFN, although without reaching statistical significance.
We explained this observation with the fact that the surgeon performing the procedure had already performed more than 30 LFNs before performing RNF, we could therefore argue if the utilisation of robotic in anti- reflux surgery could maximize its advantages in the hands of experienced surgeons.
The primary object of the study was, however, to focus on potential factors that could be related to hernia recurrence after surgery and the need of reintervention. In the literature, the rates of hiatal hernia recurrence after hiatal repair are variable and range from 1% to 7% (4). In the showed series the recurrence rate was 20%, higher than the overall recurrence rate found in the previous literature. However, looking at the characteristics of the enrolled patients we could observe how the vast majority of them had a Type 3 or Type 4 hernia at the time of surgery. In previous series the reported recurrence rate for this types of hiatal hernia were sometimes as high as 50% (4,8), and our data confirm the previous findings. Moreover, both the univariate and multivariate analyses showed how the hernia type correlates with recurrence. Our experience, however, validate the assumption that minimally invasive approaches are feasible and safe for the treatment of large hiatal hernias, as described by different authors, and that the size of the hernia it is not a contraindication to this type of surgery (4,23,25).
Another factor that correlated to recurrence was TS. This finding allow further observation on the right timing for surgery. Nowadays anti-reflux surgery is indicated laparoscopic as the standard of care for medically refractory GERD (26). Our results, however, showed a correlation between the TS. It has been previously hypothesized that recurrence and may confirm that hypothesis that recurrence of hiatal hernia may be linked to ultrastructural abnormalities at the muscular tissue. Other studies are needed to confirm this results, however we could argue that if a chronic exposition to the overpressure cause by the intrathoracic herniation of the hernia content may worsen this muscular changes the indications of surgery for GERD may change (26).
Alongside the hernia type and TS, the other factor that was found to correlate with recurrence on both univariate and multivariate analysis was obesity. It is widely known that GERD is prominent among bariatric patients. Some estimates suggest that obese patients are three times more likely than normal weight individuals to suffer from GERD (27). Currently, it is still debated if obesity could be considered a contraindication to minimally invasive hernia repair. Previous reports demonstrated no trends towards poorer outcomes in the overweight, obese, or morbidly obese patients when compared to normal weight patients, including similar complication rates and recurrence rate on long-term follow-up (28-30). Although our results did not question the feasibility and safety of minimally invasive anti-reflux surgery, preoperative BMI was an important factor that predicted recurrence after repair (31,32). In particular, our analyses showed how higher was the BMI the higher the risk of recurrence. The last factor that seemed to correlate with is the type of procedure. Although this correlation was not demonstrated in the multivariate analysis, and may be biased by the higher number of LNFs performed, we could argue if the technical advantage brought by robot may influence the recurrence rate Anyway, other prospective randomized studies are needed to better clarify this finding.
One limitation of this study include is the relatively small sample size, which could be underpowered to detect a true difference between the groups. In addition, the groups compared were heterogenous with regards to approach used. This is further limited by the fact that the study relied on retrospective review of data, as opposed to prospectively collected data.
In conclusion, minimally invasive anti reflux surgery is a valid tool to treat symptomatic GERD. Its utilisation allows to join the advantages of a minimally invasiveness, in terms of quicker recovery together with technical improvements, as a finer visualization of the hiatal region. The issue with minimally invasive techniques such as the open approach is difficulty to obtain a long lasting repair. Although, the type of hiatal hernia and obesity do not constitute absolute contraindications to minimally invasive anti-reflux surgery, proper preoperative patients selection and assessment of the right timing of surgery are crucial to draw the major benefits from this kind of surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Laparoscopic Surgery for the series “Minimally Invasive Approach for the Treatment of Gastro-esophageal Reflux Disease”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ls-20-63
Data Sharing Statement: Available at http://dx.doi.org/10.21037/ls-20-63
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at available at http://dx.doi.org/10.21037/ls-20-63). The series “Minimally Invasive Approach for the Treatment of Gastro-esophageal Reflux Disease” was commissioned by the editorial office without any funding or sponsorship. AB served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The research was approved by the local ethical committee and the informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dallemagne B, Weerts JM, Jehaes C, et al. Laparoscopic Nissen fundoplication: preliminary report. Surg Laparosc Endosc 1991;1:138-43. [PubMed]
- Granderath FA, Kamolz T, Schweiger UM, et al. Laparoscopic refundoplication with prosthetic hiatal closure for recurrent hiatal hernia after primary failed antireflux surgery. Arch Surg 2003;138:902-7. [Crossref] [PubMed]
- Yun JS, Na KJ, Song SY, et al. Laparoscopic repair of hiatal hernia. J Thorac Dis 2019;11:3903. [Crossref] [PubMed]
- Morino M, Giaccone C, Pellegrino L, et al. Laparoscopic management of giant hiatal hernia: factors influencing long-term outcome. Surg Endosc 2006;20:1011-6. [Crossref] [PubMed]
- Watson DI, Davies N, Devitt PG, et al. Importance of dissection of the hernial sac in laparoscopic surgery for large hiatal hernias. Arch Surg 1999;134:1069-73. [Crossref] [PubMed]
- Willekes CL, Edoga JK, Frezza EE. Laparoscopic repair of paraesophageal hernia. Ann Surg 1997;225:31-8. [Crossref] [PubMed]
- Landreneau RJ, Hazelrigg SR, Johnson JA. The giant paraesophageal hernia: a particularly morbid condition of the esophageal hiatus. Missouri Med 1990;87:884-8. [PubMed]
- Paul MG, DeRosa RP, Petrucci PE, et al. Laparoscopic tension-free repair of large paraesophageal hernias. Surg Endosc 1997;11:303-7. [Crossref] [PubMed]
- Maziak DE, Todd T, Pearson FG. Massive hiatus hernia: evaluation and surgical management. J Thorac Cardiovasc Surg 1998;115:53-60. [Crossref] [PubMed]
- Hashemi M, Peters JH, DeMeester TR, et al. Laparoscopic repair of large type III hiatal hernia: objective followup reveals high recurrence rate. J Am Coll Surg 2000;190:553-60; discussion 560-1. [Crossref] [PubMed]
- White BC, Jeansonne LO, Morgenthal CB, et al. Do recurrences after paraesophageal hernia repair matter? ten-year follow-up after laparoscopic repair. Surg Endosc 2008;22:1107-11. [Crossref] [PubMed]
- Wu JS, Dunnegan DL, Soper NJ. Clinical and radiologic assessment of laparoscopic paraesophageal hernia repair. Surg Endosc 1999;13:497-502. [Crossref] [PubMed]
- Zhou T, Harnsberger C, Broderick R, et al. Reoperation rates after laparoscopic fundoplication. Surg Endosc 2015;29:510. [Crossref] [PubMed]
- Skinner DB, Belsey R. Surgical management of esophageal reflux and hiatus hernia. J Thorac Cardiovasc Surg 1967;53:33-54. [Crossref] [PubMed]
- Treacy PJ, Jamieson GG. An approach to the management of paraoesophageal hiatus hernias. Aust N Z J Surg 1987;57:813-7. [Crossref] [PubMed]
- DeMeester TR, Stein JH. Minimizing the side effects of antireflux surgery. World J Surg 1992;16:335-6. [Crossref] [PubMed]
- Frazzoni M, Piccoli M, Conigliaro R, et al. Laparoscopic fundoplication for gastroesophageal reflux disease. World J Gastroenterol 2014;20:14272-9. [Crossref] [PubMed]
- Broeders JA, Rijnhart-de Jong HG, Draaisma WA, et al. Ten-year outcome of laparoscopic and conventional nissen fundoplication: randomized clinical trial. Ann Surg 2009;250:698-706. [Crossref] [PubMed]
- Ross SB, Gal S, Teta AF, et al. Late results after laparoscopic fundoplication denote durable symptomatic relief of gastroesophageal reflux disease. Am J Surg 2013;206:47-51. [Crossref] [PubMed]
- Dallemagne B, Weerts J, Markiewicz S, et al. Clinical results of laparoscopic fundoplication at ten years after surgery. Surg Endosc 2006;20:159-65. [Crossref] [PubMed]
- Morino M, Pellegrino L, Giaccone C, et al. Randomized clinical trial of robot-assisted versus laparoscopic Nissen fundoplication. Br J Surg 2006;93:553-8. [Crossref] [PubMed]
- Carlson MA, Condon RE, Ludwig KA, et al. Management of intrathoracic stomach with polypropylene mesh prosthesis reinforced transabdominal hiatus hernia repair. J Am Coll Surg 1998;187:227-30. [Crossref] [PubMed]
- Frantzides CT, Richards CG, Carlson MA. Laparoscopic repair of large hiatal hernia with polytetrafluoroethylene. Surg Endosc 1999;13:906-8. [Crossref] [PubMed]
- Arcerito M, Perez MG, Kaur H, et al. Robotic Fundoplication for Large Paraesophageal Hiatal Hernias. JSLS 2020;24:e2019.00054.
- Dallemagne B, Perretta S. Twenty years of laparoscopic fundoplication for GERD. World J Surg 2011;35:1428-35. [Crossref] [PubMed]
- Akimoto S, Nandipati KC, Kapoor H, et al. Association of Body Mass Index (BMI) with Patterns of Fundoplication Failure: Insights Gained. J Gastrointest Surg 2015;19:1943-8. [Crossref] [PubMed]
- Sanford Z, Jayaraman S, Weltz AS, et al. The role of body mass index in determining clinical and quality of life outcomes after laparoscopic anti-reflux surgery. Surg Endosc 2020;34:646-57. [Crossref] [PubMed]
- Fei L, del Genio G, Rossetti G, et al. Hiatal hernia recurrence: surgical complication or disease? Electron microscope findings of the diaphragmatic pillars. J Gastrointest Surg 2009;13:459-64. [Crossref] [PubMed]
- kimoto S, Nandipati KC, Kapoor H, et al. Association of Body Mass Index (BMI) with Patterns of Fundoplication Failure: Insights Gained. J Gastrointest Surg 2015;19:1943-8.
- Chisholm JA, Jamieson GG, Lally CJ, et al. The effect of obesity on the outcome of laparoscopic antireflux surgery. J Gastrointest Surg 2009;13:1064-70. [Crossref] [PubMed]
- D’Alessio MJ, Arnaoutakis D, Giarelli N, et al. Obesity is not a contraindication to laparoscopic nissen fundoplication. J Gastrointest Surg 2005;9:949-54. [Crossref] [PubMed]
- Lindeboom MYA, Ringers J, van Rijn PJ, et al. Gastric emptying and vagus nerve function after laparoscopic partial fundoplication. Ann Surg 2004;240:785-90. [Crossref] [PubMed]
Cite this article as: Ortenzi M, Balla A, Fontana G, Marinucci F, Reggiani A, Capomagi P, Bailetti B, Lezoche G, Guerrieri M. Factors influencing recurrence after minimally invasive treatment of hiatal hernia—a single center experience. Laparosc Surg 2020;4:39.