
How to use energy device for pure laparoscopic donor hepatectomy
Introduction
Living donor liver transplantation (LDLT) is one of main types of liver transplantation in the facts that there are not enough livers for all the potential patients with could benefit from a liver transplantation. LDLT has developed steeply over the past decades to mitigate deceased donor organ shortages and reduce mortality on waiting lists for liver transplantation mostly in countries with a scarcity of deceased donor liver graft.
Morbidity and mortality rates of living donor hepatectomy are significantly lower than hepatectomy for other disease (1). However, the safety of living liver donors is of paramount importance issue so far (2,3). In addition, the major drawbacks of living liver donation are that living donor hepatectomy is one of the major abdominal operations and occurs considerable adhesion and large operation scars in the upper abdomen. Permanent large incision scar gives young donors physical and mental stress. Surgical staffs should make the best effort to resolve the future quality of life, along with the safety of living liver donors. A minimal invasive approach to donor surgery has been developed as an alternative to solve these problems and is constantly evolving.
Laparoscopic hepatectomy has gained wide acceptance for various benign and malignant liver tumors (4). Laparoscopic hepatectomy has been carried out with minimal morbidity and mortality and with rapid recovery, the reduction of intra-operative blood loss and postoperative pain have been validated as the excellent benefits by various reviews and meta-analyses (5-8).
Blood loss is one of the main causes that affect surgical outcomes in laparoscopic liver resection (LLR). Bleeding must be a major concern for the donor surgeon in LDLT. This serious issue certainly induces the major intra-operative complications and can be one of the main causes of donor mortality and the major postoperative complications with bile leaks and hepatic failure (9-12). Hemostasis achievement during liver mobilization and parenchymal transection obviously have an effect on the successful completion of donor surgery. The main advances in reducing intra-operative bleeding has been achieved through the improvement of surgical techniques and the development in surgical instruments. Most bleeding can be encountered during transection of the liver parenchyma, which greatly affects operative time, intra-operative blood loss, blood transfusion, and postoperative morbidity and mortality (13). Introduction of various energy devices for laparoscopic surgery play an important role in reducing intra-operative blood loss during LLR.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/ls-20-22).
Methods
We started the first pure laparoscopic donor hepatectomy (PLDH) in May 2016 and a total of 79 donors received a PLDH by December 2019. All PLDHs were performed without total inflow control, such as the Pringle maneuver, but we have never experienced blood transfusion and open conversion. We would like to briefly describe our experience and several reports on clinical applications and technical tips of PLDH. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Kyungpook National University Hospital (KNUH) (No. KNUH 2020-04-054-002) which waived the need for informed consent from all individual participants.
Results
Clinical applications of energy device for PLDH
Liver mobilization
Liver mobilization is able to carry out through the dissection of ligament attachments between the liver, retroperitoneum, diaphragm, inferior vena cava (IVC), and right adrenal gland. The key feature of laparoscopic liver mobilization prevents unnecessary injuries to the IVC and hepatic veins. For safe liver mobilization, it is essential to achieve careful control of small hepatic veins with energy devices and/or clips (14).
Liver can be carefully mobilized by the optimal use of energy device. In the first step of liver mobilization, energy device is firstly used for the simple division of the ligamentum teres and falciform ligament. After dissection of these ligaments, suprahepatic IVC and hepatic veins are identified. And, we use energy devices very usefully for careful dissection of the bare area in retrohepatic space, especially for the safe separation between liver and right adrenal gland (Figure 1A). After completion of right lobe mobilization, it is important to dissect safely using an energy device and surgical clips to avoid the injury of retrohepatic IVC. Inferior right hepatic veins from retrohepatic IVC can be clearly exposed and securely ligated using an energy device and surgical clips (Figure 1B,C). Very small right inferior hepatic veins (≤1 mm) can be effectively controlled by energy devices.
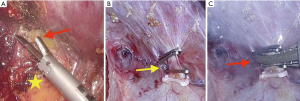
Also, in left hepatectomy, energy devices make it possible to divide left triangular ligament and gastro-hepatic ligament easily and safely.
Liver parenchymal transection
In LLR, advances in techniques and surgical instruments for parenchymal transection have facilitated a reduction of blood loss that occurred during liver resection. Control of intra-operative bleeding has been one of the significant technical issues in laparoscopic liver surgery. Various devices for the liver parenchymal transection in both open and minimally invasive liver surgery has been introduced and a few studies have reported that ultrasonic energy devices have the effect of shortening operative time and reducing postoperative complications (15,16). On the other hand, another study reported that bipolar compression devices may offer advantages over ultrasonic devices in terms of shorter total operative time, along with liver parenchymal transection time (17). However, there was no convincing data evidencing the superiority of any single technique, although there are at least 10 different techniques (18).
There are two types of devices for transection: mainly used for dissection and cutting and primarily used for hemostasis and coagulation. By a few reports, most laparoscopic living donor surgeons seem to prefer a combination of two types of devices (19-24). Table 1 shows a summary of the different types of devices for parenchymal transection in laparoscopic living donor hepatectomy. Cavitron ultrasonic surgical aspirator (CUSA Excel, Integra, USA) was mostly widely mentioned device used to transect parenchyma for LLR, especially living donor hepatectomy (25). CUSA uses ultrasonic energy to divide parenchymal tissue and keep the operative field dry by aspiration. CUSA has better performance in reducing blood loss and allows meticulous parenchymal dissection with less chance of vessel or bile duct injury. Therefore, it helps to find a precise transection plane, without damage of normal hepatic tissue (26).
Table 1
| Study | Energy device | Pringle maneuver | |
|---|---|---|---|
| Main | Combination | ||
| Samstein |
CUSA | Ligasure | No |
| Troisi |
CUSA | NA | No |
| Soubrane |
CUSA | Harmonic scalpel | NA |
| Han |
CUSA | NA | No |
| Suh |
CUSA | Thunderbeat | No |
| Kim |
CUSA | Thunderbeat | Yes |
PLDH, pure laparoscopic donor hepatectomy; CUSA, cavitron ultrasonic surgical aspirator; NA, not applicable.
Ultrasonic devices cut and coagulate using the vibration of its blades. Theoretically, ultrasonic devices seal and cut the vessels simultaneously to allow for faster tissue division. The parenchyma is also divided with secure hemostasis when the blades move in a saw-like fashion. And, small vessels (≤3 mm) can be controlled quickly and safely by ultrasonic devices (26).
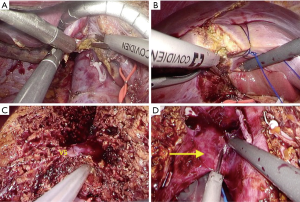
The choice of surgical technique and instruments depends entirely on the location and depth of liver transection as shown in Figure 2. The caudate lobe is easily transected using energy devices after liver mobilization (Figure 2A). Parenchymal transection in superficial layer is also performed using ultrasonic devices because the superficial layer from liver surface does not have any significant structures (Figure 2B), but in transection of deeper parenchyma, hepatic dissection device such as CUSA is more useful (27). The surface of the hepatic parenchyma up to 2 cm can be easily transected without bleeding by a Sonicision (Medtronic, Dublin, Ireland), Harmonic scalpel (Ethicon Endosurgery, Cincinnati, OH, USA) or Thunderbeat (Olympus Medical Systems Corp, Tokyo, Japan). For deeper parenchymal transection, we also used a CUSA to safely dissect intra-hepatic structures such as major hepatic veins and the branches of hepatic vein (V5 and V8) or biliary structures (Figure 2C), as with other reports (28-30). If no important structures are identified even during the parenchymal transection of deeper layer, ultrasonic device can be used to quickly transect the parenchyma, as shown in the video (Video 1). In the final stage of PLDH, including the transection of hepatic artery, portal vein and hepatic veins, energy devices allow faster dissection of remaining tissue for the rapid extraction of liver graft (Figure 2D).
Discussion
Laparoscopic donor hepatectomy requires an extremely careful and meticulous technique because small mistakes in the technique may jeopardize the donor safety. Hence, in adult-to-adult LDLT, laparoscopic donor hepatectomy was classified as IDEAL 2a, corresponding to the earliest phase of development, with the highest degree of risk because of the novelty of the procedure (31). However, the experts performing PLDH suggest that pure laparoscopic approach for donor hepatectomy will become the standard technique with the ongoing development of laparoscopic instruments and the accumulation of experience in LLR.
Many devices are now introduced for transection of the liver parenchyma in both open and laparoscopic surgery including: CUSA, Harmonic Scalpel, Thunderbeat, Sonicision, water-jet dissection, radiofrequency, microwave assisted resection, and so on. Of them, CUSA has been the most frequently used instrument for parenchymal transection during PLDH and one additional energy device is combined to reduce operation time and minimize the postoperative complications. The optimal combination and selection of energy devices depends on surgeon’s preference and familiarity.
Rhu et al. (32) reported that they used sometimes the ultrasonic shear dissector (Sonicision) alone without the CUSA to complete the dissection. This is also a surgeon’s preference and it would have been implemented based on a wealth of experience. If there are usually no major vessels found at the parenchyma up to 1–2 cm from the surface, this application of the ultrasonic shear device can be done to quickly divide the liver. But blind dissection requires great care. During parenchymal transection, meticulous dissection to preserve the major vascular branches along the resection plane and to minimize the bleeding risk, is essential for excellent results of donor surgery.
Conclusions
Donor safety is paramount in LDLT. Adopting the standardized techniques and accumulating experiences for use of energy devices allow meticulous dissection in liver resection. Therefore, precise identification of vascular or biliary structures, and elective hemostasis is the cornerstones and the basic skills of PLDH. For continued safe proliferation of PLDH, it is meaningful for the surgeons to clearly understand the advantages and limitations of energy devices. And, energy devices should be used in combination, depending on their functions and the depth of liver parenchyma. Finally, the technology of energy devices should be continuously improved to accomplish the safe PLDH, and the surgical techniques associated with energy devices must be consistently standardized and validated.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Kwang-Woong Lee and Jeong-Moo Lee) for the series “Pure Laparoscopic Donor Hepatectomy” published in Laparoscopic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/ls-20-22
Data Sharing Statement: Available at http://dx.doi.org/10.21037/ls-20-22
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls-20-22). The series “Pure Laparoscopic Donor Hepatectomy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript in ensuring that questions related to the accuracy or integrity of any part of the manuscript are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Kyungpook National University Hospital (KNUH) (No. KNUH 2020-04-054-002) which waived the need for informed consent from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Weiss A, Tapia V, Parina R, et al. Living donor hepatectomy: is it safe? Am Surg 2015;81:1101-6. [Crossref] [PubMed]
- Uchiyama H, Shirabe K, Nakagawara H, et al. Revisiting the safety of living liver donors by reassessing 441 donor hepatectomies: is a larger hepatectomy complication-prone? Am J Transplant 2014;14:367-74. [Crossref] [PubMed]
- Shin M, Song S, Kim JM, et al. Donor morbidity including biliary complications in living-donor liver transplantation: single-center analysis of 827 cases. Transplantation 2012;93:942-48. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg 2009;250:831-41. [Crossref] [PubMed]
- Nguyen KT, Marsh JW, Tsung A, et al. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg 2011;146:348-56. [Crossref] [PubMed]
- Croome KP, Yamashita MH. Laparoscopic vs open hepatic resection for benign and malignant tumors: an updated meta-analysis. Arch Surg 2010;145:1109-18. [Crossref] [PubMed]
- Mizuguchi T, Kawamoto M, Meguro M, et al. Laparoscopic hepatectomy: a systematic review, meta-analysis, and power analysis. Surg Today 2011;41:39-47. [Crossref] [PubMed]
- Gozzetti G, Mazziotti A, Grazi L, et al. Liver resection without blood transfusion. Br J Surg 1995;82:1105-10. [Crossref] [PubMed]
- Cunningham JD, Fong Y, Shriver C, et al. One hundred consecutive hepatic resections: blood loss, transfusion and operative technique. Arch Surg 1994;129:1050-6. [Crossref] [PubMed]
- Romano F, Franciosi C, Caprotti R, et al. Hepatic surgery using the Ligasure Vessel System. World J Surg 2005;29:110-2. [Crossref] [PubMed]
- Jarnagin WR, Gonen M, Fong Y, et al. improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg 2002;236:397-406. [Crossref] [PubMed]
- Castaldo ET, Earl TM, Chari RS, et al. A clinical comparative analysis of crush/clamp, stapler, and dissecting sealer hepatic transection methods. HPB 2008;10:321-6. [Crossref] [PubMed]
- Ikoma N, Itano O, Oshima G, et al. Laparoscopic liver mobilization: Tricks of the trade to avoid complications. Surg Laparosc Endosc Percutan Tech 2015;25:e21-3. [Crossref] [PubMed]
- Shander A, Hofmann A, Ozawa S, et al. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion 2010;50:753-65. [Crossref] [PubMed]
- Nanashima A, Tobinaga S, Abo T, et al. Usefulness of the combination procedure of crush clamping and vessel sealing for hepatic resection. J Surg Oncol 2010;102:179-83. [Crossref] [PubMed]
- Mbah NA, Brown RE, Bower MR, et al. Differences between bipolar compression and ultrasonic devices for parenchymal transection during laparoscopic liver resection. HPB 2012;14:126-31. [Crossref] [PubMed]
- Jagannath P, Chhabra DG, Shah R. Surgeon preferences for liver transection: is there an ideal technique? J Am Coll Surg 2010;211:141. [Crossref] [PubMed]
- Samstein B, Griesemer A, Halazun K, et al. Pure laparoscopic donor hepatectomies; Ready for widespread adoption? Ann Surg 2018;268:602-9. [Crossref] [PubMed]
- Troisi RI, Wojcicki M, Tomassini F, et al. Pure laparoscopic full-left living donor hepatectomy for calculated small-for-size LDLT in adults: proof of concept. Am J Transplant 2013;13:2472-8. [Crossref] [PubMed]
- Soubrane O, Perdigao Cotta F, Scatton O. Pure laparoscopic right hepatectomy in a living donor. Am J Transplant 2013;13:2467-71. [Crossref] [PubMed]
- Han YS, Ha H, Han JR, et al. ABO incompatible living donor liver transplantation using dual grafts and pure laparoscopic donor right hepatectomy: a case report. Medicine (Baltimore) 2018;97:e13639 [Crossref] [PubMed]
- Suh KS, Hong SK, Lee KW, et al. Pure laparoscopic living donor hepatectomy: focus on 55 donors undergoing right hepatectomy. Am J Transplant 2018;18:434-43. [Crossref] [PubMed]
- Kim KH, Kang SH, Jung DH, et al. Initial outcomes of pure laparoscopic living donor right hepatectomy in an experienced adult living donor liver transplant center. Transplantation 2017;101:1106-10. [Crossref] [PubMed]
- Cho JY, Han HS, Kaneko H, et al. Survey results of the expert meeting on laparoscopic living donor hepatectomy and literature review. Dig Surg 2018;35:289-93. [Crossref] [PubMed]
- Poon RT. Current techniques of liver transection. HPB(Oxford) 2007;9:166-73. [Crossref] [PubMed]
- Otsuka Y, Kaneko H, Cleary SP, et al. What is the best technique in parenchymal transection in laparoscopic liver resection? Comprehensive review for the clinical question on the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci 2015;22:363-70. [Crossref] [PubMed]
- Cherqui D, Laurent A, Tayar C, et al. Laparoscopic liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: midterm results and perspective. Ann Surg 2006;243:499-506. [Crossref] [PubMed]
- Kaneko H, Takagi S, Otsuka Y, et al. Laparoscopic liver resection of hepatocellular carcinoma. Am J Surg 2005;189:190-4. [Crossref] [PubMed]
- Wakabayashi G, Nitta H, Takahara T, et al. Standardization of basic skills for laparoscopic liver surgery towards laparoscopic donor hepatectomy. J Hepatobiliary Pancreat Surg 2009;16:439-44. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in morioka. Ann Surg 2015;261:619-29. [PubMed]
- Rhu J, Choi GS, Kwon CHD, et al. Learning curve of laparoscopic living donor right hepatectomy. Br J Surg 2020;107:278-88. [PubMed]
Cite this article as: Han JR, Han YS. How to use energy device for pure laparoscopic donor hepatectomy. Laparosc Surg 2020;4:38.


