
Decompression surgery of the celiac trunc—a single center experience
Introduction
Median arcuate ligament syndrome (MALS), first described in 1963 by Harjola et al. (1), results from extrinsic compression of the celiac artery by the median arcuate ligament. Clinical symptoms comprise nausea, vomiting chronic abdominal pain, postprandial pain and unintentional weight loss. The location of the pain is variable, but most commonly spotted in the epigastrium. MALS is a rare condition, which predominantly prevails in mid aged women who have a lean body habitus (2).
Hypotheses for the etiology of MALS are diverse (3) and range from visceral ischemia (4), neuropathic compression (5) to vascular steal phenomena, caused by collateral vessels (6). Incidental findings also demonstrated that celiac trunc compression or stenosis may furthermore be present in a relatively high number (7,3%) of asymptomatic patients (7).
In case of celiac trunc stenosis or even occlusion, an increased blood flow through the pancreaticoduodenal arcade from the superior mesenteric artery (SMA) may be sufficient to compensate an otherwise insufficient arterial blood flow to the liver and stomach. However, subsequent arterial hyper-perfusion of the pancreaticoduodenal arcade, may foster the development of visceral artery aneurysms within this circulatory system (8). Decompression of the celiac trunc and reestablishment of sufficient arterial blood flow through the celiac route is hence mandatory before any endovascular intervention (e.g., embolization of the aneurysm) on the pancreaticoduodenal route may be considered, since occlusion of the entire arcade may result in acute ischemia induced liver injury. It is furthermore critical to recognize celiac trunc compression in patients requiring a liver transplant, since the arterial reconstruction during transplant comprises dissection of the hepatic artery and frequent division of the collateral circulation including the gastroduodenal artery (9).
The current literature predominantly provides case reports or small case series which describe both open and laparoscopic techniques for celiac trunc decompression (3). Currently, universally valid diagnostic criteria do not exist, however doppler ultrasound, CT angiograph and angiographic evaluation of possible changes in celiac velocities which are altered by inspiration and expiration, are commonly applied.
It is hence our aim to raise awareness of this rare disease, provide an insight in to our patient collectives, namely those with MALS as well as a group of liver transplant recipients who required celiac trunc decompression at the time of transplant.
Methods
Medical datasets of patients diagnosed with celiac artery compression syndrome were evaluated retrospectively for the years 2016 to 2019. For this survey, the diagnosis code I77.4 was used to recover patient data stored in our institutional data base. Additionally, patients undergoing liver transplantation in 2019 were reviewed for coeliac trunc decompression procedures during transplantation. Anonymized patient details, clinical histories, operative details and outcomes were retrospectively reviewed. SPSS and Excel was used for statistical analysis. This retrospective study was approved by the institutional review board.
Minimally invasive operative technique for median arcuate ligament decompression surgery
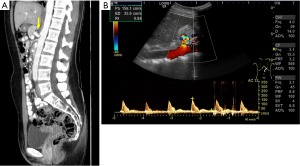
The detailed laparoscopic surgical procedure for median arcuate ligament decompression, is provided in Video 1. As an example, we demonstrate the case of a 31-year-old female patient (Figure 1), who reported of nausea, vomiting and postprandial pain for the last 6 months. Doppler ultrasound examination and CT angiography revealed a compression of the coeliac trunc, caused by the median arcuate ligament (Figure 2). The patient was scheduled for minimally invasive surgery, following informed consent.
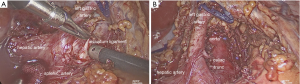
In short, surgery was performed under general anesthesia and in French position. Following a team timeout, and under sterile conditions a 12 mm camera trocar was inserted into the abdomen through the umbilicus. A capno-peritoneum of 12 mm Hg was maintained constantly through the entire operation. After explorative laparoscopy using a 30° optic which revealed no contraindications for the planned procedure, a 10 mm and a 5 mm trocar were placed into the right and left upper abdomen, respectively. Another 5 mm trocar for suction and retraction could additionally be placed into the left subcostal region. For liver retraction a Nathanson liver retractor was inserted through a sub xiphoidal incision.
In a first step, the minor omentum was transected and the left gastric artery was dissected free from the accompanying vein. The coeliac trunc trifurcation, comprising the left gastric artery, the hepatic artery and the splenic artery should be identified and dissected up to the perivascular sheet (Figure 1).
In a next step, first ramifications of the arcuatum ligament located on the celiac trunc were transected using laparoscopic scissors and bipolar forceps. Another valuable instrument widely applicable for this type of operation are ultrasonic sheers, which might facilitate delicate and fast preparation along the coeliac trunc, up to the front of the abdominal aorta. It is of importance, that all diaphragmatic muscle fibers anterior to the aorta are dissected free at a length of 4–5 cm to prevent perhaps a recurrence of celiac trunc compression. Finally, all trocars were removed and the incisions were closed using PDS 3-0 interrupted sutures. Skin closure was performed by intracutaneous running sutures. Postoperative doppler ultrasound examinations were performed on postoperative day three, before discharge of the patient.
Median arcuate ligament decompression during liver transplantation
In liver transplant candidates with celiac trunc compression, adequate arterial blood flow has to be established prior hepatectomy and subsequent liver transplantation. Screening for celiac trunc compression is performed by preoperative CT angiography. Arterial blood flow was intraoperatively measured by doppler ultrasound and the Medistim MiraQ Flow measurement system. In case of celiac decompression, a subsequent arterial blood flow of more than 200 mL/second, including the expiratory phase, was rated sufficient for transplant and reperfusion.
Results
Minimally invasive decompression of the median arcuate ligament
Twelve patients were diagnosed with (I77.4) coeliac trunc compression syndrome between 01/2016 and 12/2019. Patient demographics are listed in Table 1. Mean patient age was 51±19 years and MALS had a higher incidence in women (women: n=9, 64%; men n=5, 36%). To date n=2 (16,7%) were treated by minimally invasive decompression surgery. One patient presented with a classical MALS (Figure 2), and one patient additionally presented with an aneurysm of the pancreaticoduodenal arcade which was coiled three days after coeliac trunc decompression surgery (Figure 3). Mean operative time was 50±6 minutes. No abdominal drainages were used.
Table 1
| Nr. | Gender | Age | Diagnostic procedures | Treatment |
|---|---|---|---|---|
| 1 | F | 46 | Doppler US; CT angiography, upper GI endoscopy, angiography | No treatment, mild symptoms |
| 2 | M | 80 | Doppler US; CT angiography, upper GI endoscopy, | No treatment, higher age, mild symptoms |
| 3 | M | 57 | Doppler US; CT angiography, upper GI endoscopy, angiography | Indication for laparoscopic decompression, patient in follow up |
| 4 | F | 47 | Doppler US; CT angiography, upper GI endoscopy, angiography | Indication for laparoscopic decompression, patient lost in follow up |
| 5 | F | 70 | Doppler US; CT angiography, upper GI endoscopy | Indication for laparoscopic decompression, patient in follow up |
| 6 | F | 53 | Doppler US; CT angiography, upper GI endoscopy | Indication for laparoscopic decompression, patient lost in follow up |
| 7 | F | 40 | Doppler US; CT angiography, upper GI endoscopy | Mild stenosis, no treatment, mild symptoms |
| 8 | M | 61 | Doppler US; CT angiography, upper GI endoscopy | Indication for laparoscopic decompression, patient lost in follow up |
| 9 | F | 79 | Doppler US; CT angiography, upper GI endoscopy | No treatment, higher age, mild symptoms |
| 10 | F | 20 | Doppler US; CT angiography, upper GI endoscopy | Indication for laparoscopic decompression, patient in follow up |
| 11 | M | 30 | doppler US; CT angiography, upper GI endoscopy | Indication for laparoscopic decompression, patient in follow up |
| 12 | F | 31 | doppler US; CT angiography, upper GI endoscopy | Laparoscopic decompression |
| 13 | F | 44 | doppler US; CT angiography, upper GI endoscopy, aneurysm of pancreaticoduodenal arcade | Laparoscopic decompression, angiographic coiling of aneurysm |
| 14 | M | 20 | doppler US; CT angiography, upper GI endoscopy | Mild stenosis, no treatment, mild symptoms |
To date 2 patients (nr 13 and nr 14) received successful laparoscopic decompression surgery. Six patients presented with mild symptoms and hence did not qualify for surgery. Another six patients refused a surgical procedure and preferred a short interval surveillance strategy. Two patients did not meet their follow up appointments and were hence lost in follow-up. MALS, median arcuate ligament syndrome.
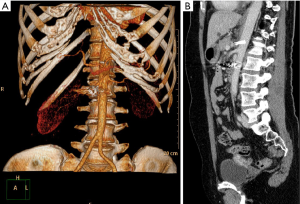
The remaining n=10 (83.3%) patients either presented with mild clinical symptoms (n=6, 60%) and therefore did not qualify for surgery, or refused surgery and preferred a clinical follow up strategy instead (n=4, 40%). With regard to clinical surveillance group, two patients refused further monitoring and were hence lost in follow-up.
Our diagnosis treatment algorithm for patients with MALS comprised the following: After (I) clinical examination and survey of the medical history, (II) duplex sonography, (III) CT angiography and (IV) upper GI endoscopy is performed. In case of severe clinical symptoms due to stenosis of the coeliac trunc a minimally invasive release of the median arcuate ligament is recommended and the patient is scheduled for surgery after informed consent. Postoperative duplex sonography is generally performed on postoperative day three, which is the regular day of discharge. If clinical symptoms are resolved, which was the case in our first two patients, treatment is completed. In case of persistent symptoms, angiography and pressure gradient examination is performed and the patient is evaluated for further angioplasty, stent placement or surgical vascular reconstruction.
Decompression of the median arcuate ligament during liver transplantation
In 2019 a total number of n=32 orthotopic liver transplants were performed at our institution. Demographic patient data and indications for liver transplantation are listed in Table 2 and Figure 4, respectively. The average waiting time for liver transplant recipients was 6.4±6.4 month and the average Model for End Stage Liver Disease (MELD) at time of enrolment and transplant was 18±8 and 20±9 respectively. Leading indications for transplant were alcohol induced liver cirrhosis (ASH, 44%) as well as cirrhosis from non-alcoholic steatohepatitis (NASH, 16%) and primary sclerosing cholangitis (PSC, 13%). Based on preoperative CT screening, four patients (12.5%) were identified with a ligamentous compression of the celiac trunc with otherwise normal arterial anatomy (Michels Type 1). In these cases, decompression of the celiac artery was performed as a first step of the transplant procedure. It is of importance to facilitate adequate arterial blood flow prior hepatectomy, since this potential time-consuming maneuver should not be performed during the an-hepatic phase of liver transplantation. All celiac trunc decompressions were successful. Graft reperfusion was performed as “artery first” and subsequent portal vein reperfusion. Arterial flow was controlled by intraoperative doppler ultrasound and MiraQ Flow measurement (233±25 mL/min). Overall morbidity, defined as Clavian Dindo grade 3b or higher, was 18,75% (n=6). Sixty-day mortality was 3.2% (n=1). Morbidity attributed to celiac trunc decompression was 0%.
Table 2
| Number of LTX | n=32 |
|---|---|
| Number of celiac trunc decompression | n=4 (12.5%) |
| Age | 60±7 |
| Gender | f=22 (31.3%); m=10 (68.7%) |
| Waiting time (month) | 6.4±6.4 |
| MELD at listing | 18±8 |
| MELD at transplant | 20±9 |
MELD, Model For End-Stage Liver Disease.

Discussion
Minimally invasive decompression of the celiac trunc has been increasingly accepted as the gold standard treatment for MALS. First reported in 1995 by Loffeld et al. (10), the laparoscopic approach bears several patient benefits, including smaller incisions and decreased postoperative morbidity, and in parallel provides the surgeon with an improved surgical view of the operation field (11). More recently, the application of robotic assisted techniques (12) have been introduced to the minimally invasive surgical world (13), which potentially are capable to even outperform the laparoscopic techniques in terms of technical (enhanced 3-D vision and intraabdominal maneuverability) and operator based (tremor elimination, operators comfort) criteria (14). In the shade of these modern surgical techniques, endoscopic retroperitoneal and open surgical approaches are gradually tapered from the daily surgical business (15). This is especially true for open surgical techniques which are mainly reserved for cases which require more than just ligamental decompression.
The diagnosis of MAL syndrome, because of its resemblance to other abdominal disorders, commonly is a diagnosis of exclusion. Currently no standardized diagnostic criteria exist, however Kim et al. recently provided a well-arranged algorithm for diagnosis and management of MALS, which hence has also been applied to our patients (14). This comprises a clinical patients history workup, followed by ultrasound CT scan and upper GI endoscopy. In case of normal findings diagnostic strategies are accompanied by a more detailed CT or MRT angiography, as well as a CT angiography of the celiac trunc with detailed pressure gradient measurements. In case of celiac stenosis, a minimally invasive decompression is aspired. If symptoms are resolved after surgery patients are followed up by doppler ultrasonography. In case of symptoms` persistence an alternative approach comprising angioplasty and stenting is taken into account. The high reticence in regard to decompression surgery, furthermore highlights the fact that commonly patients are torn between a high degree of physical and psychological affliction and fear of the surgical intervention.
The liver is unique in that it is supplied by dual blood inflow. While the portal vein provides the major part of hepatocyte’s blood consumption, the bile ducts solely depend on arterial blood flow from the hepatic artery (9). Flawless revascularization during transplant is key to successful postoperative graft function and necessary to prevent ischemic type bile-duct lesions (ITBL). Arterial reconstruction depends on the anatomical conditions and commonly involves the anastomosis of the donor hepatic artery to the recipient’s hepatic artery just at the origin of the gastroduodenal artery. “Artery first” reperfusion, performed as a standard in our institution, has been demonstrated to be beneficial (16,17) since biliary epithelial cells are more susceptible to warm ischemia than hepatocytes (18). It is hence crucial that proper surgical measures are taken, which in some circumstances include the decompression of the celiac trunc, to facilitate adequate arterial blood supply at time of reperfusion.
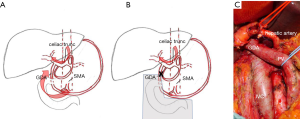
With regard to celiac trunc compression or occlusion, one more group of individuals require special attention. Patients scheduled for operations which comprise the division of the (GDA) gastroduodenal artery (e.g., pancreatoduodenectomies, visceral aneurysm repair etc..), must be screened carefully, since an intraoperative division of the GDA may stop an otherwise vital retrograde arterial blood flow to the liver (Figure 5) and ultimately result in ischemic liver failure.
In the era of minimally invasive surgery different surgical disciplines benefit from each other (19). Our laparoscopic operations were performed by a team of both hepatobiliary surgeons and vascular surgeons. We strongly believe that fundamental laparoscopic skills and a detailed knowledge of the vascular anatomy and a faithful preoperative diagnostic workup (20) is necessary for successful treatment of celiac compression syndromes.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls.2020.03.08). RS serves as an unpaid editorial board member of Laparoscopic Surgery from Oct 2019 to Sep 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Institutional Review Board of University Clinic Leipzig and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Harjola PT. A Rare Obstruction of the Coeliac Artery. Report of a Case. Ann Chir Gynaecol Fenn 1963;52:547-50. [PubMed]
- Trinidad-Hernandez M, Keith P, Habib I, et al. Reversible gastroparesis: functional documentation of celiac axis compression syndrome and postoperative improvement. Am Surg 2006;72:339-44. [Crossref] [PubMed]
- Kohn GP, Bitar RS, Farber MA, et al. Treatment options and outcomes for celiac artery compression syndrome. Surg Innov 2011;18:338-43. [Crossref] [PubMed]
- Marable SA, Molnar W, Beman FM. Abdominal pain secondary to celiac axis compression. Am J Surg 1966;111:493-5. [Crossref] [PubMed]
- Vaziri K, Hungness ES, Pearson EG, et al. Laparoscopic treatment of celiac artery compression syndrome: case series and review of current treatment modalities. J Gastrointest Surg 2009;13:293-8. [Crossref] [PubMed]
- Delis KT, Gloviczki P, Altuwaijri M, et al. Median arcuate ligament syndrome: open celiac artery reconstruction and ligament division after endovascular failure. J Vasc Surg 2007;46:799-802. [Crossref] [PubMed]
- Park CM, Chung JW, Kim HB, et al. Celiac axis stenosis: incidence and etiologies in asymptomatic individuals. Korean J Radiol 2001;2:8-13. [Crossref] [PubMed]
- Terayama T, Tanaka Y, Soga S, et al. The benefits of extrinsic ligament release for potentially hemodynamically unstable pancreaticoduodenal arcade aneurysm with median arcuate ligament syndrome: a case report. BMC Surg 2019;19:50. [Crossref] [PubMed]
- Jurim O, Shaked A, Kiai K, et al. Celiac compression syndrome and liver transplantation. Ann Surg 1993;218:10-2. [Crossref] [PubMed]
- Loffeld RJ, Overtoom HA, Rauwerda JA. The celiac axis compression syndrome. Report of 5 cases. Digestion 1995;56:534-7. [Crossref] [PubMed]
- Takach TJ, Livesay JJ, Reul GJ Jr, et al. Celiac compression syndrome: tailored therapy based on intraoperative findings. J Am Coll Surg 1996;183:606-10. [PubMed]
- Lafaro KJ, Fong Y. Robotic liver surgery from the patient’s perspective. Laparosc Surg 2019;3:57. [Crossref]
- Jaik NP, Stawicki SP, Weger NS, et al. Celiac artery compression syndrome: successful utilization of robotic-assisted laparoscopic approach. J Gastrointestin Liver Dis 2007;16:93-6. [PubMed]
- Kim EN, Lamb K, Relles D, et al. Median Arcuate Ligament Syndrome-Review of This Rare Disease. JAMA Surg 2016;151:471-7. [Crossref] [PubMed]
- Goodall R, Langridge B, Onida S, et al. Median arcuate ligament syndrome. J Vasc Surg 2019; [Crossref] [PubMed]
- Polak WG, Porte RJ. The sequence of revascularization in liver transplantation: it does make a difference. Liver Transpl 2006;12:1566-70. [Crossref] [PubMed]
- Noun R, Sauvanet A, Belghiti J. Appraisal of the order of revascularization in human liver grafting: a controlled study. J Am Coll Surg 1997;185:70-3. [Crossref] [PubMed]
- Noack K, Bronk SF, Kato A, et al. The greater vulnerability of bile duct cells to reoxygenation injury than to anoxia. Implications for the pathogenesis of biliary strictures after liver transplantation. Transplantation 1993;56:495-500. [Crossref] [PubMed]
- Sucher E, Sucher R. Minimally invasive liver surgery: a field is maturing. Laparosc Surg 2019;3:34. [Crossref]
- Takahara T, Wakabayashi G, Hasegawa Y, et al. Preoperative work-up for donor main consideration for laparoscopic surgery. Laparosc Surg 2020;4:7.
Cite this article as: Sucher E, Sucher R, Geisler A, Guice H, Doss M, Gockel I, Seehofer D, Branzan D. Decompression surgery of the celiac trunc—a single center experience. Laparosc Surg 2020;4:25.


