Laparoscopic splenectomy: conventional technique and challenges
Introduction
The laparoscopic approach for splenectomy was first described in 1991 (1) and it immediately demonstrated several advantages when compared to conventional surgery. Indeed, carrying out a splenectomy by open approach requires a wide surgical incision, as the spleen is located deeply in the upper left quadrant of the abdomen. Since that time, different approaches have been proposed with patients placed in supine, lateral or prone position.
We have standardized our laparoscopic approach, describing an easily reproducible technique. It is realized by transabdominal route with a patient in a lateral position just as for a laparoscopic adrenalectomy (2), thereby offering the surgeon anatomical landmarks that can be easily identified and an early approach to the splenic artery.
Indications
Surgical indications for splenectomy remain relatively limited in terms of frequency. The indications are mainly based on medical treatment failure; laparoscopy has little place in the management of acute or traumatic spleens. Indeed, laparoscopy is contraindicated in cases of active bleeding, and the orientation towards conservative treatments prevails today in cases of post-traumatic spleens.
The main indication for splenectomy is idiopathic thrombocytopenic purpura (ITP). It has the advantage of presenting in patients who have a small spleen. The other indications reported in Table 1 include all indications (3,4).
Table 1
| Indication | Incidence |
|---|---|
| Autoimmune low platelet counts ITP (Idiopathic Thrombocytopenic Purpura) | 50–65% |
| Spherocytosis | 9–15% |
| Neoplasm/lymphoma | 5–27% |
| Autoimmune hemolytic anemia & AIDS | 7–12% |
| Others including benign tumor | 5–10% |
Pre-operative workup
The required pre-operative assessment before laparoscopic splenectomy is relatively limited. Biologically, the main restriction can be the presence of anemia. This will require pre-operative correction by transfusions of red blood cells to obtain a hemoglobin level at least between 9 and 10 g/dL. This value is necessary because this procedure can be complicated by sudden intra-operative bleeding.
A common problem encountered when performing this surgery is that of a relatively low platelet count. Nevertheless, if the lower limits are often 30 to 40,000 platelets/mL for a surgical procedure, there is no such threshold in the pre-operative assessment of splenectomy for thrombocytopenic purpura. It is possible to operate without platelet transfusion with platelet counts of 1,000 platelets/mL: experience has shown that platelet count is restored very quickly immediately following a splenectomy, with a platelet count after 2 hours in order of 30,000/mL (5).
Abnormalities of coagulation should be corrected pre-operatively. Conventional contraindications for laparoscopy remain applicable.
Pre-operative imaging remains fundamental for the surgeon, but often raises issues of interpretation. The reference examination is the abdomen and chest CT scan. This provides a measurement of the spleen’s size. The transverse and longitudinal measurements do not give a precise idea of its real volume. Indeed, the size of the spleen is often underestimated and splenomegaly is frequently encountered unexpectedly during surgical procedure. The diameter or length of the spleen underestimate its actual size and in the absence of a 3D reconstruction, volumetric analysis may be a more useful measurement. Any spleen larger than 1 liter can potentially cause intra-operative problems. The second important element of pre-operative imaging is an accurate description of the vascularization. This allows the surgeon to ascertain the presence of a single splenic artery or to identify an early division that will lead to control of the two branches separately. Venous return analysis may identify portal hypertension associated with increased surgical risk. Finally, CT scanners now makes it possible to precisely identify accessory spleens, the removal of which is imperative in order to effectively treat an ITP. The incidence of accessory spleens is 6% to 15%, usually localized in the main hilum of the spleen. However, the accessory spleens, usually under 8 mm in 60% of cases, might be localized anywhere in the omentum, along the pancreas, or even in the pelvis (6).
Surgical technique
Many surgical approaches have been described for performing splenectomies. As far as the installation of the patient is concerned, the anterior, anterolateral, and anteroposterior approaches have been described. In the same way, “hand-assisted” approaches or one-trocar-related approaches have been described (7); however as the authors write, “…description is based only on case reports or small series… and firm conclusions cannot yet be drawn…”.
The aim is to propose a simple and reproducible technique for most splenectomies, in lean or overweight patients, for small or large spleens. This standardization is the best guarantee of surgical success.
All procedures are performed under general anesthesia with muscle relaxation and controlled ventilation. Patients are monitored carefully during surgery, with special attention to oxygen saturation and end-tidal CO2 levels.
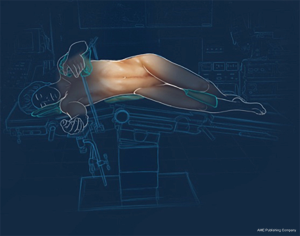
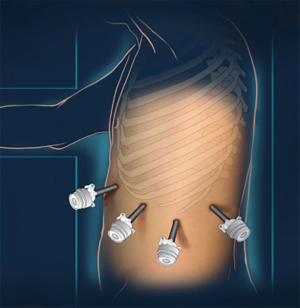
A lateral position on the right side is required (Figure 1). The procedure is started with the introduction of the first trocar under direct vision on the anterior axillary line, just under the costal margin. In case of a large spleen, the trocars have to be inserted lower than the costal margin (Figure 2). CO2 pressure is regulated at 12 mmHg for the whole procedure. A 10 mm 30° laparoscope is introduced to enable intra-abdominal exploration.
The surgical procedure is divided into five stages:
- release of the omentum at the lower edge of the spleen and mobilization of the left colic angle;
- access to the lesser sac and release of pancreatic caudal arterial branches;
- dissection and control of the splenic pedicle;
- release of short gastric vessels, and
- posterior release of the lieno-renal ligament and posterior attachments.
Release of the omentum at the lower edge of the spleen and mobilization of the left colic angle
After insertion of the optic, two other 10 mm ports are inserted for introduction of the atraumatic graspers, hook, instrument with peanut swab, and scissors. The surgical procedure begins by freeing the adhesions of the left colic angle to the anterior wall of the abdomen, as these adhesions create a curtain which alters the dissection axis of the instruments. Furthermore, removing the adhesions may be necessary if a fourth trocar is required in the case of a large spleen or of adipose patients.
The key to success is to start the procedure by dissecting the splenorenal ligament starting at the inferior pole of the spleen, and to cut the peritoneal reflection at a distance of 1 cm from the spleen.
Access to the lesser sac and release of pancreatic caudal arterial branches
The release of the lower pole of the spleen allows access to the lesser sac. The dissection is immediately in contact with the upper and anterior surface of the pancreas. It is necessary to release the tail of the pancreas to obtain a safe distance between the hilum of the spleen and the pancreas. This usually leads to the identification of a communicating branch between the splenic artery and the distal part of the pancreas. It is mandatory to identify and control this artery so as to prevent its tearing, which would result in an arterial wound in contact with the tail of the pancreas. This would require either a suture or an electro-surgical procedure that could damage the pancreas and be the cause of a postoperative pancreatic fistula or a distal pancreatitis.
Dissection and control of the splenic pedicle
The hilum of the spleen does not contain elements other than the artery and the splenic vein. The dissection of this area is done step by step. The splenic artery is usually easier to identify because of the beating near its route. It will be identified and dissected over a length of 1 to 2 cm. In case of difficulty, it is possible to apply a first clip upstream of the hilum and dissect the artery progressively and distally. The artery will be controlled by the different means available to the surgeon: ligature by non-absorbable thread sutured to the artery to avoid slippage, absorbable or non-absorbable clip application, stapler. Although it is possible to do so, it is not recommended to use fusion devices (LigasureTM; Ultrasound dissectors…) to control the splenic artery because of its diameter and possible atheromatous lesions that would impair the quality of tissue fusion. After the first vascular control of the artery, the splenic vein will be progressively dissected. The lack of arterial vascularization of the spleen will quickly decrease the flow of the splenic vein, enabling its safe dissection. When it is sagging, its diameter is only 1 or 2 mm and it can be controlled easily with clips. Completeness of the spleen’s vascular control can then be checked as the ischemic nature of the splenic tissue is easy to identify.
Separate ligation of the splenic artery and the splenic vein is the least risky option to prevent a delayed arteriovenous fistula. However, in certain situations, it may be appropriate to perform a “block” control of this vascular pedicle. It is then possible to use a linear stapler to perform simultaneous vascular control of the splenic artery and vein. The main disadvantages of this simultaneous ligation are the increased risk of post-operative arteriovenous fistula, but also the risk of lesion to the tail of the pancreas in case of imperfect dissection of the latter, and incidentally the absence of emptying of the blood reserve of the spleen in the portal circuit after first ligation of the artery.
Release of short gastric vessels
The last vascular elements to be controlled are the short gastric vessels. The dissection of this area corresponds to that performed during the preparation of the stomach to perform a fundoplication. It should be carried out close to the spleen to avoid any damage to the gastric wall. These small vascular pedicles, 2 or 3 in number, often contain a slightly larger artery at their upper part. For tissue control, ultrasonic or fusion systems achieve a safe, rapid, and effective dissection.
Once this zone is released, all the vascular attachments of the spleen are released. The spleen is suspended only by its posterior peritoneal attachments.
Posterior release of the lieno-renal ligament and posterior attachments
The spleen will be slightly raised with an atraumatic retractor, and its posterior attachments released directly by section and electrocoagulation. The dissection will not extend at a distance because there is no other retroperitoneal attachment.
After complete release, the spleen falls spontaneously into the right side of the abdomen.
Extraction of the spleen
The spleen cannot be extracted easily by small incisions. It is recommended to first place it in an extraction bag inside the abdomen. This bag will be progressively brought to the level of the parietal wall by the widest incision. The spleen will then be gradually broken up in the bag by graspers and extracted fragment by fragment. This avoids a more significant scar. Fragmentation is commonly accepted, because for pathological examination it is not necessary to maintain an ‘en bloc’ spleen.
Discussion
The laparoscopic approach to splenectomy has become a standard for all teams performing this surgery regularly. However, it is important to identify the difficulties and potential pitfalls to minimize the risk of operational accidents.
In addition to the conventional pre-operative assessments, CT scan pre-operative imaging, with injected arterial and venous phases, is highly recommended in order to define the operative strategy and identify potential difficulties beforehand.
One difficulty may be related to the tail of the pancreas being in close proximity of the hilum of the spleen. It is sometimes embedded into the spleen and it is difficult to perform a dissection of the vascular pedicle without lesion of the tail of the pancreas. This difficulty can be quite easily identified pre-operatively by a precise analysis of pre-operative images. It allows surgeons to adapt their strategy for controlling the vascular pedicle, deciding whether it is preferable to use a clip after elective dissection of the vessels, to carry out a ligation section in one piece by a linear stapler, or to control the vessels separately by conventional suture. The main goal is of course to prevent any damage to the pancreatic tail resulting in a pancreatic fistula or a pancreatitis.
The precise analysis of the pre-operative images makes it possible to identify precisely the vascular distribution of the splenic pedicle. In some cases, there is an early division of the splenic artery and splenic vein, making it necessary to dissect these two pedicles electively. This may in some cases facilitate partial splenectomy. Elective vascular control of each of these pedicles is required to carry out the splenectomy.
Another difficulty is caused by underestimating the spleen size. A volumetric assessment would easily identify spleens over 1 liter in volume. It should be noted that a diameter over 10 cm exposes the surgeon to a potentially difficult procedure. However, as it has been well demonstrated, the size of the spleen itself, even in cases of splenomegaly, is not a contraindication to the laparoscopic approach (8). The limit would be in the order of 2 kg, i.e., 2 liters in volume, corresponding to measurements in excess of 20–22 cm, which would be considered as massive splenomegaly (9). In these cases, however, laparoscopy could still be proposed, to prepare the upper and lower dissections of the spleen and to limit the requirement for large surgical incisions.
In patient management for ITP, the main risk is intervention failure by recurrence of the pathology. Recurrence is mainly caused by the wound of the spleen capsula when performing the surgical procedure which exposes splenic tissue dissemination in the omentum, thereby creating splenosis by implantation of micro- or neo-spleen in the omentum. The outcomes of the management of these pathologies by laparoscopy are comparable to those of open surgery, with complete remission of the pathology in 60% of cases, partial remission in 22% of cases, and a failure in 14% of cases (10). It is therefore recommended to avoid any manipulation of the spleen that may expose rupture of the capsule during the surgical procedure or during its extraction.
Intra-operative complications of this surgery are rare, and relate mainly to hemorrhage which occurs in about 5% of cases, most often by lesion of a large vessel. Improper handling of a large spleen can lead to capsular rupture resulting in permanent bleeding that impairs the dissection. The only solution to prevent this discomfort is to quickly apply a clip on the artery to reduce bleeding. However, this can lead to a conversion. Interestingly, and despite the occasional difficulty of carrying out this procedure, the conversion rate in the laparoscopic approach of the spleen remains low, in the order of 3% (4). The main predictor of conversion is the size of the spleen. Any spleen sized over 1 liter is likely to cause a conversion to laparotomy.
At the end of the procedure, surgical drainage is usually no longer necessary. However, some authors propose to put in place this drainage, especially if there is a risk of pancreatic fistula. The incidence of drainage found in the literature is usually about 7%. Nonetheless, drainage is not necessary to prevent complications such as postoperative bleeding. Aspirative drainage may be indicated.
The post-operative period is usually straightforward. The patient recovers rapidly and, in our experience, leaves the hospital the day after surgery.
The main post-operative risk is that of portal thrombosis (11). This is observed in 3% to 10% of cases, equivalent to that observed during splenectomy by laparotomy. This complication is resolved spontaneously in most cases; in 20% of cases however there is a risk of developing a portal cavernoma (12). To prevent this risk, it is imperative to prescribe a one-month course of subcutaneous heparin. This requirement was confirmed by the EAES Consensus Conference in 2008 (13).
In conclusion, the laparoscopic approach to splenectomy is appropriate in all indications. In larger spleen cases, the preparation by releasing the lower and upper poles will facilitate the procedure by limiting the size of the laparotomy.
Laparoscopy for splenectomy brings the same benefits to the patient as other laparoscopic procedures, for reducing post-operative pain, associated morbidity and hospitalization duration, and for its excellent cosmetic result. In addition, however, muscle preservation allows personal activities to be resumed earlier and reduces the risks of hernia or parietal muscle weakness linked to large lumbotomies.
Laparoscopy is today the reference approach for this surgery.
Acknowledgments
The author gratefully acknowledges Isabelle Petty for contributing to this manuscript by providing language editing and administrative assistance.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (H. Leon Pachter) for the series “Laparoscopic Surgery of the Liver and Spleen” published in Laparoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls.2020.03.01). The series “Laparoscopic Surgery of the Liver and Spleen” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Delaitre B, Maignien B. Splenectomy by the laparoscopic approach. Report of a case. Presse Med 1991;20:2263. [PubMed]
- Marescaux J, Mutter D, Wheeler MH. Laparoscopic right and left adrenalectomies. Surgical procedures. Surg Endosc 1996;10:912-5. [Crossref] [PubMed]
- Katkhouda N, Hurwitz MB, Rivera RT, et al. Laparoscopic splenectomy: outcome and efficacy in 103 consecutive patients. Ann Surg 1998;228:568-78. [Crossref] [PubMed]
- Radkowiak D, Zychowicz A, Lasek A, et al. 20 years' experience with laparoscopic splenectomy. Single center outcomes of a cohort study of 500 cases. Int J Surg 2018;52:285-92. [Crossref] [PubMed]
- Wu Z, Zhou J, Pankaj P, et al. Laparoscopic splenectomy for immune thrombocytopenia (ITP) patients with platelet counts lower than 1 × 109/L. Int J Hematol 2011;94:533-8. [Crossref] [PubMed]
- Unver Dogan N, Uysal II, Demirci S, et al. Accessory spleens at autopsy. Clin Anat 2011;24:757-62. [Crossref] [PubMed]
- Targarona EM, Lima MB, Balague C, et al. Single-port splenectomy: Current update and controversies. J Minim Access Surg 2011;7:61-4. [PubMed]
- Targarona EM, Espert JJ, Balagué C, et al. Splenomegaly should not be considered a contraindication for laparoscopic splenectomy. Ann Surg 1998;228:35-9. [Crossref] [PubMed]
- Terrosu G, Baccarani U, Bresadola V, et al. The impact of splenic weight on laparoscopic splenectomy for splenomegaly. Surg Endosc 2002;16:103-7. [Crossref] [PubMed]
- Berends FJ, Schep N, Cuesta MA, et al. Hematological long-term results of laparoscopic splenectomy for patients with idiopathic thrombocytopenic purpura: a case control study. Surg Endosc 2004;18:766-70. [Crossref] [PubMed]
- Ikeda M, Sekimoto M, Takiguchi S, et al. High incidence of thrombosis of the portal venous system after laparoscopic splenectomy: a prospective study with contrast-enhanced CT scan. Ann Surg 2005;241:208-16. [Crossref] [PubMed]
- Vecchio R, Cacciola E, Cacciola RR, et al. Portal vein thrombosis after laparoscopic and open splenectomy. J Laparoendosc Adv Surg Tech A 2011;21:71-5. [Crossref] [PubMed]
- Habermalz B, Sauerland S, Decker G, et al. Laparoscopic splenectomy: the clinical practice guidelines of the European Association for Endoscopic Surgery (EAES). Surg Endosc 2008;22:821-48. [Crossref] [PubMed]
Cite this article as: Mutter D. Laparoscopic splenectomy: conventional technique and challenges. Laparosc Surg 2020;4:29.