
Preoperative work-up for donor main consideration for laparoscopic surgery
Introduction
Living-donor liver transplantation (LDLT) has become a widely accepted therapeutic alternative to deceased donor liver transplantation (DDLT) with equivalent and promising outcomes. Laparoscopic approaches to liver surgery have evolved over the years and minor resections (segmental and anatomic) are now considered to be a standard practice in selected patients with equivalent and often superior results as compared to open liver resections.
Pure laparoscopic donor hepatectomy (PLDH) has become increasingly accepted in the current era of minimally invasive surgery. After the first successful report of laparoscopic left lateral sectionectomy during adult-to-child LDLT in 2002, the procedure is now recommended in highly specialized centers by international consensus (1). PLDH in adult-to-adult LDLT was first reported in 2013 (2). However, the adoption of laparoscopic liver resection (LLR) to donor hepatectomy has been slow and some concerns have increased regarding donor’s safety. The Louisville Consensus Conference in 2008 and the Second International Consensus on Laparoscopic Liver Surgery in 2014 reported that laparoscopic donor major hepatectomy is in the earliest phase of development with an unclear benefit/risk ratio and uncertainty regarding the long-term outcomes of donors and recipients (1). According to an expert panel statement for PLDRH during the 26th World Congress of the International Association of Surgeons, Gastroenterologists and Oncologists (IASGO) in 2016, only skillful surgeons with enough experience in both LLR and LDLT should perform PLDRH (3). In this paper, we review the evaluation of donors and work up of PLDRH in several high-volume laparoscopic liver surgery centers in Asia.
Method
We reviewed the donor evaluation process and work up of laparoscopic donor hepatectomy in Asian high-volume centers that performed laparoscopic liver surgery, with reference to published papers. Specifically, we examined the selection criteria for right lobe grafts, which were frequently utilized in adult-to-adult LDLT. Moreover, we analyzed the weight of the graft and the frequency of anomalies of the vessels (hepatic artery, portal vein, and hepatic vein) and the bile duct in PLDRH.
Results
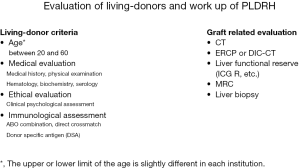
Preoperative evaluation issues of PLDRH were shown in Figure 1. The detailed evaluations of each high-volume center are as follows.
Iwate Medical University School of Medicine (4)
Age of the living donors was adults from 20 to 65 years, and all of the living donors had comprehensive medical evaluations, including cardiovascular, pulmonary, and renal assessments. Donor candidates were routinely counselled by a psychiatrist about their voluntary intention to donate. The transplant coordinator routinely provided precise information about LDLT to donors and confirmed legal relationships between the donors and recipients. Donor candidates with systemic diseases such as diabetes mellitus, hypertension, or psychiatric diseases were strictly excluded. Donor livers were evaluated by four-phase multidetector computed tomography (MD-CT) and drip-infusion cholangiography computed tomography (DIC-CT) with three-dimensional reconstruction. We had two requirements for hepatic function of the living donor: an indocyanine green retention rate at 15 minutes (ICG R15) of less than 10 as a measurement of hepatic reserve and a liver/spleen (L/S) ratio of greater than 1.1 as an assessment for fatty liver. If the ICG R15 was slightly higher than 10, we performed asialoglycoprotein receptor imaging to examine the functional reserve of the donor liver. If fatty liver was suspected by the L/S ratio, the donor candidate received nutrition support guidance and we arranged to have the liver functional reserve re-assessed after at least 3 months. When the L/S ratio and ICG R15 met our criteria, the donor candidate waited with proper diet and exercise for as long as the recipient’s condition allowed. In some cases, liver biopsy was performed to rule out steatohepatitis, non-alcoholic steatohepatitis (NASH), or histological abnormalities (5).
Our criteria for graft selection were a remnant left liver volume of greater than 35% of the donor whole liver and an estimated graft weight of more than 0.7% of the recipient’s body weight. In the recipient operation, if the volume of the congested liver was estimated to be greater than 100 mL using a volume analyzer software, we planned to reconstruct the tributaries of the middle hepatic vein in the right lobe graft without using the middle hepatic vein itself. Donor evaluation was based on the criteria approved by the Institutional Review Board of Iwate Medical University School of Medicine.
Asan Medical Center (6,7)
In Korea, each donation was approved by the ethics committee of the local authority and by the Korean Network for Organ Sharing (KNOS), which is affiliated with the Korean Ministry of Health. In addition to the preoperative evaluation necessary for general anesthesia, the pretransplantation evaluation of the living donors included standard liver function tests, doppler ultrasonography, triphasic liver CT, magnetic resonance cholangiopancreatography (MRCP), liver biopsy, and ICG R15. In the initial period, donors who had single and relatively long segments in the right hepatic artery, right portal vein, and a single right hepatic duct were selected for PLDRH.
Seoul National University College of Medicine (8-10)
Magnetic resonance spectroscopy (MRS) was preoperatively performed for the evaluation of the donor fatty liver. Liver biopsies were routinely performed for potential donors with fat fraction >8–10% as determined by MRS and other factors including older age, and elevated body mass index (BMI). If necessary, these potential donors were enrolled in a short-term weight reduction program. It was an absolute requirement to procure a right lobe graft only if the estimated remnant liver volume was over 30% of the whole liver.
All donors had undergone preoperative MRCP, which has replaced intraoperative cholangiography in this center since 2009. More recently, an ICG near-infrared fluorescence camera system for real-time cholangiography was introduced in March 2016. Initially, PLDRH was only performed in selected donors who had no anomalies of the portal vein or bile duct. However, since March 2016, no special selection criteria were applied once the technique had become sufficiently established.
Seoul National University Bundang Hospital (11)
The donor selection criteria for PLDRH in the initial phase included an expected graft weight of more than 1.0% of the recipient weight, a remnant left liver volume of greater than 35% of the donor whole liver, no unsuitable vascular or biliary variation for resection and anastomosis, normal laboratory test results, and the recipient being medically stable.
Sumsung Medical Center (12-14)
All donors were required to have an expected remnant liver volume of more than 30% after right lobe grafts. At the beginning of PLDRH adoption, only patients under 60 years with an expected remnant liver volume of greater than 35% of the whole liver were selected. Additionally, any anatomical variations that might have required sophisticated techniques for the laparoscopic procedure were excluded, and only type 1 portal veins and type 1 bile ducts were included.
The anomaly frequency of the vessels and the bile duct, and the graft weight in each institution were shown in Table 1. Regarding the graft weight, the largest grafts were procured by pure laparoscopy in the most highly experienced institutions. The surgical skill to mobilize the large right lobe without damaging the right lobe graft may have depended on the number of PLDRH procedures previously carried out in the center. In the introductory phase of PLDRH, only donors with no anatomical variation were selected in even these high-volume centers.
Table 1
| Asian high-volume centers | PV anomaly (%) | HA anomaly (%) | V5, V8, IRHV reconstruction (%) | Bile duct anomaly (%) | Graft weight (g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Recent | Initial | Recent | Initial | Recent | Initial | Recent | Initial | Recent | |||||
| Seoul National University Bundang Hospital (n=33) ( |
NA | 20.6 | 38.2 | NA | 750 | |||||||||
| Seoul National University College of Medicine (n=115) ( |
14 | 5.9 | NA | NA | 38.1 | 39.2 | 700 | 736.5 | ||||||
| Asan Medical Center (n=3) ( |
0 | – | 0 | – | 100 | – | 0 | – | 556.7 | – | ||||
| Samsung Medical Center (n=100) ( |
5 | NA | 45 | 18 | 712 | |||||||||
| Iwate Medical University School of Medicine (n=17) ( |
0 | 17.6 | 58.8 | 47.1 | 668 | |||||||||
PLDRH, pure laparoscopic donor right hepatectomy; PV, portal vein; HA, hepatic artery; V5, segment V; V8, segment VIII; IRHV, inferior right hepatic vein; NA, not applicable.
Postoperative biliary complications were associated with difficulties assessing bile duct anatomy during PLDH. To prevent biliary complications such as leakage and/or stenosis, it is important to close the stump securely. The laparoscopic suturing technique is necessary for closing the stump of the bile duct definitively while avoiding bile leakage and stenosis of the residual bile duct. On the other hand, the bile duct was cut with a safe distance from the residual bile duct to prevent stenosis. As a result of this, Suh et al. reported that the percentage of surgeries with multiple bile duct openings was significantly higher in PLDRH than in open donor right hepatectomy (9).
Careful attention must be given to perform PLDRH safely for living donors who have portal vein anomalies, as well as to perform open donor right hepatectomy. Lee et al. reported a subgroup analysis of donor who underwent PLDRH in which the rate of major complications was 4.7% in the initial group but 0% in the most recent group (10). The completion of about 60 PLDRHs per medical center is sufficient to standardize the procedure. Suh et al. also reported that modern technical developments such as three-dimensional laparoscopes and real-time, ICG, near-infrared fluorescence cameras have brought about substantial benefits (9). In Korea, the use of flexible three-dimensional laparoscopy and ICG near-infrared fluorescence cholangiography might improve the safety and outcome of PLDRH in donors with anatomical, vascular, and biliary variants.
Summary
Meticulous and vigilant donor evaluation is the most important and necessary aspect of LDLT. Therefore, there should not be any compromise in the process of evaluating and selecting a living donor. Extensive experience in LLR and LDLT is necessary to introduce PLDRH in medical centers, and precise evaluations of anatomical variants and hepatic reserve are essential to perform PLDRH safely.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Kwang-Woong Lee and Jeong-Moo Lee) for the series “Pure Laparoscopic Donor Hepatectomy” published in Laparoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls.2019.12.02). The series “Pure Laparoscopic Donor Hepatectomy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Soubrane O, Perdigao Cotta F, Scatton O. Pure laparoscopic right hepatectomy in a living donor. Am J Transplant 2013;13:2467-71. [Crossref] [PubMed]
- Han HS, Cho JY, Kaneko H, et al. Expert panel statement on laparoscopic living donor hepatectomy. Dig Surg 2018;35:284-8. [Crossref] [PubMed]
- Takahara T, Wakabayashi G, Nitta H, et al. The first comparative study of the perioperative outcomes between pure laparoscopic donor hepatectomy and laparoscopy-assisted donor hepatectomy in a single institution. Transplantation 2017;101:1628-36. [Crossref] [PubMed]
- Hasegawa Y, Kawachi S, Shimazu M, et al. Discontinuation of living donor liver transplantation for PSC due to histological abnormalities in intraoperative donor liver biopsy. Am J Transplant 2007;7:2204-7. [Crossref] [PubMed]
- Kim KH, Jung DH, Park KM, et al. Comparison of open and laparoscopic live donor left lateral sectionectomy. Br J Surg 2011;98:1302-8. [Crossref] [PubMed]
- Kim KH, Kang SH, Jung DH, et al. Initial outcomes of pure laparoscopic living donor right hepatectomy in an experienced adult living donor liver transplant center. Transplantation 2017;101:1106-10. [Crossref] [PubMed]
- Hong SK, Lee KW, Choi Y, et al. Initial experience with purely laparoscopic living-donor right hepatectomy. Br J Surg 2018;105:751-9. [Crossref] [PubMed]
- Suh KS, Hong SK, Lee KW, et al. Pure laparoscopic living donor hepatectomy: focus on 55 donors undergoing right hepatectomy. Am J Transplant 2018;18:434-43. [Crossref] [PubMed]
- Lee KW, Hong SK, Suh KS, et al. One Hundred fifteen cases of pure laparoscopic living donor right hepatectomy at a single center. Transplantation 2018;102:1878-84. [Crossref] [PubMed]
- Lee B, Choi Y, Han HS, et al. Comparison of pure laparoscopic and open living donor right hepatectomy after a learning curve. Clin Transplant 2019;33:e13683 [Crossref] [PubMed]
- Kwon CHD, Choi GS, Kim JM, et al. Laparoscopic donor hepatectomy for adult living donor liver transplantation recipients. Liver Transpl 2018;24:1545-53. [Crossref] [PubMed]
- Park J, Kwon DCH, Choi GS, et al. Safety and risk factors of pure laparoscopic living donor right hepatectomy: comparison to open technique in propensity score-matched analysis. Transplantation 2019;103:e308-16. [Crossref] [PubMed]
- Rhu J, Choi GS, Kim JM, et al. Feasibility of total laparoscopic living donor right hepatectomy compared with open surgery: comprehensive review of 100 cases of the initial stage. J Hepatobiliary Pancreat Sci 2019; [Epub ahead of print]. [PubMed]
Cite this article as: Takahara T, Wakabayashi G, Hasegawa Y, Nitta H, Katagiri H, Kanno S, Umemura A, Sasaki A. Preoperative work-up for donor main consideration for laparoscopic surgery. Laparosc Surg 2020;4:7.


