
Laparoscopic treatment of Caroli’s disease
Introduction
Caroli’s disease (CD) belongs to the group of congenital intrahepatic bile duct dilatation diseases (IHBDD). CD is a rare hepatobiliary disorder characterized by malformations of the intrahepatic medium and larger bile ducts without pancreatic biliary junction dysfunction, resulting in non-obstructive ductal dilatation, with focal or multifocal segmental involvement of the liver (1). It was exhaustively described for the first time in 1958, by the French gastroenterologist Jacques Caroli, who attempted its classification and defined this condition as a congenital malformation of the intrahepatic bile ducts, characterized by mono- or bilobar segmental polycystic dilatation (2). This disorder induces biliary stasis, leading to unspecific related symptoms and complications, mainly represented by lithiasis, cholangitis, localized or systemic infections, secondary biliary cirrhosis and bile duct cancers.
The CD is a rare disorder, with a prevalence of approximate less than one in 1,000,000 inhabitants, more frequently affecting female with a ratio male-female of 1:1,8. The onset of the disease in 80% of cases is before 30 years of age (3). There are multiple genetic and acquired factors supposed to contribute to the pathogenetic mechanisms of CD. Even if the specific etiology of this disorder is still unknown, many studies have suggested that the more frequent mode of inheritance is autosomal recessive, in particular in association with the polycystic kidney disease. The supposed cytogenetic mechanism could be an unbalanced translocation between chromosome 3 and 8, producing in particular the loss of distal 3p and/or gain of 8q. Other embryological events could be involved in the etiopathogenesis of CD, resulting in an abnormal remodeling of bile ducts (3). The abnormal or incomplete development of embryonic biliary ductal plate can be localized or involve the entire intrahepatic biliary tree (3). Jacques Caroli reported the two forms of the disease: simple form is characterized by localized cystic dilatation of the intrahepatic bile ducts without other concomitant conditions, and the complex form termed Caroli’s syndrome is associated with coexisting congenital intrahepatic fibrosis and/or even cirrhosis, portal hypertension, renal cystic congenital disease (4).
The conditions associated to CD affecting kidneys are the autosomal polycystic kidney disease, both the dominant and recessive forms (ARPKD), medullary sponge kidney, medullary cystic disease. In those conditions, renal tubular ectasia or cystic lesions of kidney are similar to the lesions of the bile ducts. The common pathogenetic factor is the recessive mutation of PKHD1 gene (5). This gene is expressed in the fetal biliary system and contributes to the bile duct embryogenesis. In patients with ARPKD or other similar kidney diseases the presence of CD should be suspected and the application of proper imaging techniques is encouraged, allowing early detection and prevention of severe complications. In patients with ARPKD, CD can be found in 30% of cases (6).
Clinical presentation
Even though the disease can be asymptomatic, the clinical presentation usually occurs within the first three decades of life. There is not a specific clinical scenario of presentation. The most frequent symptoms are: recurrent fever, cholestasis, jaundice, pruritus, pancreatitis, weight loss, epigastrium and right hypochondrium pain.
Recurrent acute cholangitis is the most frequent presentation of the disease that can reduce significantly the quality of life of these patients. The expected survival for patients with recurrent cholangitis is 5–10 years (5).
Complications of the disease are: liver abscesses, intra- or extra-hepatic lithiasis and cholangiocarcinoma, with a frequency of 2.5–16% of patients. Therefore, the risk of developing this malignancy is estimated to be 100-fold increase in CD (1,3). The proposed factors promoting carcinogenesis are chronic inflammation and the prolonged exposure to carcinogenetic substances contained in the bile (1). This condition could cause a chronic injury to liver function, secondary biliary cirrhosis and the clinical consequences of portal hypertension, as reported in Caroli’s syndrome. Blood tests usually show elevation of alkaline phosphatase, direct bilirubin, leukocytosis and, in more severe cases, liver function impairment (3). In patients with Caroli’s syndrome thrombocytopenia and leucopenia are frequently present due to the presence of portal hypertension (4). Associated kidney disease occurs in 60% of the cases with different degree of function impairment. Other associated pathologic findings are: portal vein cavernomatosis, pulmonary hypertension with arteriovenous fistula, pulmonary fibrosis, congenital heart disease, Joubert’s syndrome, amyloidosis, Laurence-Moon Beidl syndrome and neurofibromatosis (5,7).
Diagnosis and imaging
The diagnosis of CD can be very challenging giving clinical presentation with unspecific findings and symptoms (8). When asymptomatic the diagnosis can be incidental, based on blood tests and imaging findings (1). The differential diagnosis between CD and advanced cases of intrahepatic biliary stone disorder can be particularly difficult. Nevertheless, in case of choledocholithiasis without gallbladder stones the presence of CD should be suspected and properly investigated (3,8).
Characteristic imaging findings of CD are cystic dilatation in continuity with the biliary tree. The more frequently utilized tools are: ultrasonography (US), computed tomography (CT) and magnetic resonance cholangiopancreatography (MRCP). In addition, other diagnostic methods are: endoscopic retrograde cholangiopancreatography (ERCP), percutaneous transhepatic cholangiography (PTC), Technetium TC 99m colloid sulfur and DISIDA scintigraphy.
In particular, common finding of US and CT are liver cysts, intrahepatic lithiasis and portal radicles surrounded by dilated intrahepatic bile ducts (9). MRCP is a non-invasive diagnostic tool that can precisely visualize the entire biliary tree and is the preferred detection method for CD. Commonly, the MRCP pathognomonic radiological finding is the enhancing fibrovascular bundles along the dilated bile ducts (“central dot sign”) (3).
ERCP and PTC allow an accurate diagnosis, but the incidence of serious complications such as sepsis, bile leakage, bleeding and death can reach 3% of procedures and for this reason are suggested only with therapeutic intent.
Liver biopsy can be useful providing information about the presence of fibrotic changes or cirrhosis, epithelial dysplasia and cancer (8).
Finally, the conclusive diagnosis is achieved through the evidence of radiologic “central dot sign”, saccular or fusiform segmental dilatations connected with normal distal and/or proximal bile ducts associated in some cases with liver fibrosis at liver biopsy (10).
The diagnosis of biliary duct cancers can be challenging because the imaging finding of CD can mask the presence of cancer and for this reason diagnosis of cancer is frequently incidental at final pathologic examination after surgical intervention for CD.
Pathological features
The definitive diagnosis of CD is confirmed by pathological examination. In surgical specimens studies, CD is characterized by non-obstructive, segmental and subsegmental cystic dilatations of the intrahepatic bile ducts, fibrosis and stenosis of bile ducts, intracystic lithiasis, intraluminal protrusions of patent fibrovascular peripheral branches (3). This finding corresponds to the radiologic description of “central dot sign”.
Biliary epithelium is usually irregular and abraded with a normal surrounding hepatic parenchyma or with fibrotic changes in case of recurrent cholangitis or associated congenital hepatic fibrosis (1). In addition, persistence of ductal plate-like structures can be detected confirming the supposed pathogenesis with defective remodeling of the ductal structures (8).
Differential diagnosis
Differential diagnosis for CD should consider all the biliary diseases with chronic inflammation and cholestasis. In detail, primary sclerosing cholangitis (PSC), recurrent pyogenic cholangitis, polycystic liver disease, choledochal cysts, biliary papillomatosis and chronic obstructive biliary disease are the most frequent conditions with clinical and radiological findings similar to CD. However, PSC is frequently characterized by more isolated and fusiform dilatations and it is associated with inflammatory bowel disease in about 70% of the patients. In polycystic liver disease, the hepatic cysts are rarely in continuity with the bile ducts and the biliary tree is usually normal. Choledochal cystic disease is usually limited to the extrahepatic bile ducts without intrahepatic involvement. The differential diagnosis with recurrent pyogenic cholangitis is more difficult for the presence of sepsis with intra- and extrahepatic dilatation commonly observed also in CD (3).
Classification systems
Over the years, many classifications of congenital intrahepatic bile duct dilatations have been proposed. Guntz et al. radiological subclassification of IHBDD described three types: the type I (including CD) in case of “grape bunch-like” saccular dilatation of peripheral intrahepatic bile ducts alternating with normal ones, the type II with fusiform communicating dilatation of large intrahepatic bile ducts and the type III with saccular communicating dilatation of large intrahepatic bile ducts (10,11).
The classification of biliary tree malformations proposed by Todani et al. is the more frequently adopted, it is based on both morphologic features and location of the abnormalities and describes five different categories: type I includes spherical or fusiform dilatation of the entire extrahepatic biliary tract; type II that corresponds to the extrahepatic supraduodenal biliary diverticulum; type III is the cystic dilatation of the intramural duodenal segment of the common bile duct, termed choledochocele; type IV when there are multiple extrahepatic biliary cysts, either alone or in association with multiple large intrahepatic biliary cysts; CD corresponds to the group V (12,13).
Treatment
The choice of treatment depends on the extension of the disease, the associated conditions, the clinical course of the disorder and patient’s performance status. Monolobar disease is more frequent (80% of cases) and usually located in the left lobe, while right monolobar disease is extremely rare (5). Patients suffering from monolobar disease are potentially curable by resective surgery, that demonstrated to be effective, obtaining good long-term results with complete and long-lasting relief from symptoms (1). Basic principle of resective surgery should include the complete resection of any pathologic bile duct, in order to avoid the development of recurrent disease or cancer. The risk of carcinogenesis and recurrence of cholangitis increase over time, for these reasons surgical radical treatment should be performed as soon as possible and with the purpose of removing all pathological bile ducts (14). Surgery results are significantly related to pre-operative management and the presence of infective complications before surgery. Endoscopic or percutaneous invasive procedures prior to surgery are associated with an increased risk of overall complications and high rate of post-operative infections (10,14).
However, it was reported in literature that about 20% of the patients undergoing surgery need subsequent radiologic or endoscopic procedures for biliary complications or for recurrent disease of contralateral lobe. Notably, the clinical course of localized CD is extremely different from the bilobar one, despite the similar etiopathogenetic mechanism. While localized CD may be successfully treated with radical surgical resection, the bilobar disease has a poor prognosis, since it is characterized by the onset of several severe complications with complex and demanding treatment. Specifically, recurrent cholangitis and cirrhosis are related to a mortality rate of 20–40% and morbidity of 44–80% due to the progressive deterioration of liver function and severe infective complications.
Patients affected by extensive bilobar disease are candidates for liver transplantation, which is indicated in patients developing progressive hepatic disease with liver decompensation, but also in extensive asymptomatic disease (7). CD represents less than 0.2% of all liver transplantation’s indications (10). Nevertheless, the results of transplantation are worsened in case of congenital hepatic fibrosis, recurrent cholangitis and incidental cholangiocarcinoma, because of the high recurrence rate (3).
Medical therapy for patients with CD includes ursodeoxicolic acid and appropriate and tailored antibiotic therapy for cholangitis. In case of biliary obstruction, abscesses and lithiasis, biliary drainage can be required. The choice of endoscopic, radiological approach is based on the location of the disease and local medical expertise (3). However, these non-surgical procedures are associated with high risk of infectious complications and high recurrence rate, increasing morbidity and mortality with a delay in a potential curative surgical intervention.
Laparoscopic surgery
Minimally invasive liver surgery (MILS) has been increasingly widespread in the recent years for treatment of benign and malignant diseases. From this perspective, many studies have demonstrated benefits and safety of minimally invasive approach in comparison with open surgery (15). In particular, the laparoscopic approach became the standard of care in left lateral sectionectomy, but nowadays it can be safely applied for more complex resections (16).
Up to now, the most common surgical approach to CD is open resection. Indeed, scientific literature on MILS focused on CD is considerably lacking in this topic, even though left hepatectomy and left lateral sectionectomy are the most frequent treatments for localized disease (15).
The scarce experience in MILS can be explained by the rarity of the disease and its complex presentation involving segmental or lobar bile ducts (14). The presence of severe complications as recurrent cholangitis and diffuse hepatolithiasis are often a limitation to MILS approach, moreover complex intraoperative biliary procedures such as cholangioscopy and bilio-enteric anastomosis are additional factors limiting MILS approach. Secondly, according to the etiopathogenesis of the disease, the only effective resective treatment is the complete excision of the involved segments, leading to the necessity of more complex surgery with anatomical resection.
However, MILS is considered to be feasible, reaching excellent long-term results and with a low rate of conversion, also in CD.
In addition to the well-known laparoscopic advantages, patients with impairment of liver function should benefit from MILS, with a lower risk of post-hepatectomy complications (15). Furthermore, the MILS could be suggested as primary approach in patients for whom, because of the complexity of CD, liver transplantation could be eventually required.
According to literature, MILS for CD requires standard laparoscopic equipment, but particular attention should be paid to intraoperative bile ducts examinations. Although, cholangioscopy and cholangiography can be useful to confirm the presence of residual stones and ensure the complete resection of pathological bile ducts (Figures 1,2) (17).
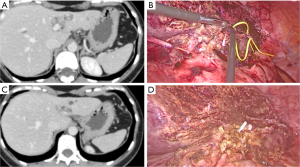
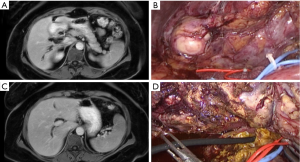
Intraoperative biopsy with frozen section histological examination is suggested in case of suspected malignancy (18). In patients with incidental intraoperative diagnosis of cholangiocarcinoma, radical margin free resection and regional lymphadenectomy are required with open or MILS approach (14).
We have reviewed the existing literature in MEDLINE database of peer review papers published during the period 2005–2018 included 243 liver resections for CD (19-22). Among these published papers only six more recent (2009–2018) reported MILS resections for CD (7 left lateral hepatic sectionectomies, 7 left hepatectomies, 1 segmentectomy; 1 right anterior sectionectomy) describing technical aspects, feasibility, safety, short- and long-term outcome. The total number of reported cases of MILS for CD is only 16 (Table 1). In these cases, CD was limited to the left liver. The reported conversion rate was 0–12.5% and the rate of complication was 6.2%. Finally, there are few reported data about long term follow-up, in particular authors did not report the recurrence rate of CD and of hepatolithiasis.
Table 1
| Authors | Patients | Operative procedures | Conversion | Complications | Follow-up |
|---|---|---|---|---|---|
| Abayie |
1 | LLLHS | 0 | 0 | – |
| Chen |
1 | LLH | 0 | 0 | 18 months |
| Mabrut |
8 | LLLHS (n=5); LLH (n=2); S5 (n=1) | 1 (LLLHS) | – | – |
| Hwang |
1 | LRAS | 0 | Fluid collection, conservatively treated | – |
| Di Giuro |
4 | LLH | 0 | – | – |
| Boni |
1 | LLLHS | 0 | 0 | – |
MILS, minimally invasive liver surgery; CD, Caroli’s disease; LLLHS, laparoscopic left lateral hepatic sectionectomy; LLH, laparoscopic left hepatectomy; S5, segmentectomy V; LRAS, laparoscopic right anterior sectionectomy.
Conclusions
CD is a rare condition with variable clinical complexity due to the extension of the disease, the localization of pathological bile ducts and the presence of complications. The radical surgery is the treatment of choice for localized disease and in these cases MILS can have an important therapeutic role, in particular for left located CD. MILS approach to CD requires high expertise in liver surgery and in complex biliary procedures.
Data of the literature are still lacking in long-term results and more studies are necessary to confirm the short and long-term results.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Francesco Ardito) for the series “The Role of Minimally Invasive Liver Surgery to Treat Hepatic Benign Disease” published in Laparoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls.2019.11.01). The series “The Role of Minimally Invasive Liver Surgery to Treat Hepatic Benign Disease” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yamaguchi T, Criastaudi A, Kokudo T, et al. Surgical treatment for monolobular Caroli’s disease - Report of a 30-year single center case series. BioScience Trends 2018;12:426-31. [Crossref] [PubMed]
- Longmire WP Jr, Mandiola SA, Gordon HE. Congenital cystic disease of the liver and biliary system. Ann Surg 1971;174:711-26. [Crossref] [PubMed]
- Yonem O, Bayraktar Y. Clinical characteristics of Caroli’s disease. World J Gastroenterol 2007;13:1930-3. [Crossref] [PubMed]
- Wang ZX, Li YG, Wang RL, et al. Clinical classification of Caroli’s disease: an analysis of 30 patients. HPB (Oxford) 2015;17:278-83. [Crossref] [PubMed]
- Moslim MA, Gunasekaran G, Vogt D, et al. Surgical management of Caroli’s disease: single center experience and review of the literature. J Gastrointest Surg 2015;19:2019-27. [Crossref] [PubMed]
- Kumar A, Akselrod D, Prikis M. Caroli Disease Revisited: A Case of a Kidney Transplant Patient With Autosomal Polycystic Kidney Disease and Recurrent Episodes of Cholangitis. Transplant Proc 2019;51:541-4. [Crossref] [PubMed]
- Habib S, Shakil O, Couto OF, et al. Caroli’s disease and orthotopic liver trans-plantation. Liver Transpl 2006;12:416-21. [Crossref] [PubMed]
- Yadav P, Adhikari S, Pandit N, et al. Caroli’s disease: a diagnostic challenge. Int Surg J 2018;5:3750-3. [Crossref]
- Choi BI, Yeon KM, Kim SH, et al. Caroli disease: central dot sign in CT. Radiology 1990;174:161-3. [Crossref] [PubMed]
- Mabrut JY, Kianmanesh R, Nuzzo G, et al. Surgical management of congenital intrahepatic bile duct dilatation, Caroli’disease and syndrome. Ann Surg 2013;258:713-21. [Crossref] [PubMed]
- Guntz P, Coppo B, Lorimier G, et al. Single-lobe Caroli's disease. Anatomoclinical aspects. Diagnostic and therapeutic procedure. Apropos of 3 personal cases and 101 cases in the literature. J Chir (Paris) 1991;128:167-81. [PubMed]
- Todani T, Watanabe Y, Narusue M, et al. Congenital bile duct cysts: Classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg 1977;134:263-9. [Crossref] [PubMed]
- Banks JS, Saigal G, D’Alonzo JM. Choledochal malformations: surgical implications of radiologic findings. AJR Am J Roentgenol 2018;210:748-60. [Crossref] [PubMed]
- Fahrner R, Dennler SGC, Dondorf F, et al. Liver resection and transplantation in Caroli disease and syndrome. J Visc Surg 2019;156:91-5. [Crossref] [PubMed]
- Goh BK, Chan CY, Lee SY, et al. Laparoscopic liver resection for tumors in the left lateral liver section. JSLS 2016; [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Ramia JM, Palomoque A, Muffak K, et al. Indications and therapeutical options in hepatolithiasis. Rev Esp Enferm Dig 2006;98:597-604. [Crossref] [PubMed]
- Chen CB, Hu WD, Zhao WW, et al. Laparoscopic hepatectomy for the treatment of Caroli's disease: a case report. Ann Surg Treat Res 2018;94:162-5. [Crossref] [PubMed]
- Bockhorn M, Malagò M, Lang H, et al. The role of surgery in Caroli’s disease. J Am Coll Surg 2006;202:928-32. [Crossref] [PubMed]
- Lendoire J, Barros Schelotto P, Alvarez Rodriguez J, et al. Bile duct cyst type V (Caroli's disease): surgical strategy and results. HPB (Oxford) 2007;9:281-4. [Crossref] [PubMed]
- Mabrut JY, Partensky C, Jaeck D, et al. Congenital intrahepatic bile duct dilatation is a potentially curable disease: long-term results of a multi-institutional study. Ann Surg 2007;246:236-45. [Crossref] [PubMed]
- Kassahun WT, Kahn T, Wittekind C, et al. Caroli’s disease: liver resection and liver transplantation. Experience in 33 patients. Surgery 2005;138:888-98. [Crossref] [PubMed]
- Abayie AO, Nyarko KM, Loza N, et al. Laparoscopic Liver Resection in a Case of Asymptomatic Elderly Patient with Caroli Syndrome. J Gastrointest Cancer 2018; [Epub ahead of print]. [PubMed]
- Hwang DW, Han HS, Yoon YS, et al. Totally Anatomic Laparoscopic Right Anterior Sectionectomy. J Laparoendosc Adv Surg Tech A 2012;22:913-6. [Crossref] [PubMed]
- Di Giuro G, Lainas P, Franco D, et al. Laparoscopic left hepatectomy with prior vascular control. Surg Endosc 2010;24:697-9. [Crossref] [PubMed]
- Boni L, Dionigi G, Rovera F, et al. Laparoscopic left liver sectoriectomy of Caroli's disease limited to segment II and III. J Vis Exp 2009; [Crossref] [PubMed]
Cite this article as: Ruzzenente A, Alaimo L, Conci S, Bagante F, Campagnaro T, Ciangherotti A, Guglielmi A. Laparoscopic treatment of Caroli’s disease. Laparosc Surg 2020;4:4.


