
Laparoscopic radiofrequency ablation (RFA) of liver tumors
Introduction
Thermal ablation of hepatic tumors remains a cornerstone of local-regional treatment for both primary liver cancers and metastatic tumors to the liver. While ablation is second in efficacy to surgical hepatic resection, ablation can aim toward curative intent in many circumstances when properly deployed. Thus, thermal or heat ablation using radiofrequency ablation and lately, microwave ablation, are part of the standard of care armamentarium for which hepatobiliary surgeons should be skilled at performing. Ablation has the additional advantage in that it can be performed with minimal invasiveness and thus is most commonly performed percutaneously using either ultrasound or computed tomography (CT) guidance by interventional radiologists. However, circumstances will still arise whereupon ablation will need to be performed surgically and can thus be pursued utilizing laparoscopy in these clinical scenarios. This review will summarize the indications, benefits, and potential pitfalls for laparoscopic ablation of liver tumors.
Indications and technical considerations
Expected efficacy and safety are the primary considerations for deciding upon the need for ablation of liver tumors as oncologic efficacy favors resection over ablation (discussed later) whereas in some cases ablation can provide nearly similar oncologic benefit but with greater safety depending on patient factors and hepatic related factors. If the patient is not a resection candidate, the indications for laparoscopic ablation of liver tumors must consider several factors. These factors include cancer type, underlying liver disease, relative tumor number, tumor size, anatomical location, and whether concomitant resection of another liver tumor is required. The primary consideration is whether there are contraindications to the patient undergoing percutaneous radiologic guided ablation. The most common contraindications to percutaneous approaches are location of the tumor such that it is adjacent to other structures such as the gall bladder, colon, stomach, or diaphragm—structures which may become traumatized during a percutaneous approach. Clearly if an additional tumor needs to be resected, then ablation can be done in the same setting. Tumors should be 3 cm or less in size as both randomized controlled trials and cohort based retrospective studies have shown that recurrence rate is significantly elevated, regardless of cancer type, if tumors exceed this size (1-3). Tumor number, like resection, does not have a clear contraindication, but as the number of ablations are increased, the likelihood of recurrence becomes higher as does potential risk of hepatic related complications (2,4,5). Contraindications to ablation include tumors that are adjacent to hilar structures, particularly the bile ducts, as either incomplete ablation or damage to the bile ducts are the result which can be devastating to liver function. Additionally, if tumors are adjacent to major hepatic or portal vein branches are ablated, this could result in incomplete ablation due to “heat sink effect” secondary to the high volume of circulating blood which can cool the adjacent tissue or can result in thrombosis due to vessel wall injury.
While tumors less than 3 cm are candidates for ablation, tumors must also be large enough to be identified while performing intraoperative liver ultrasonography. Hepatobiliary surgeons must be proficient in hepatic ultrasound in order to perform intraoperative ablation. The identification of hepatocellular carcinomas (HCCs) can be more difficult in the presence of cirrhosis. B mode ultrasound coupled with availability of colored Doppler where necessary to identify biliary vs. vascular structures is utilized along with a flexible laparoscopic 10 mm probe. RFA or microwave probes are then placed under ultrasound guidance into the tumor to aim for a 1-cm ablation margin if possible. For purposes of this review, the electrical-physical properties for the various manufacturers radiofrequency and microwave platforms will not be discussed. However, both radiofrequency and microwave technologies using medium frequency alternating current (350–500 kHz) or electromagnetic waves (300 MHz–300 GHz), respectively, achieve a thermal temperature increase for the targeted tissue of greater than 60 °C to achieve coagulative necrosis for the ablation zone. Ablation probes are chosen depending on the manufacturer’s guidelines for tumor size and expected margin of ablation. Once ablation has been performed, ablated tissue is indistinguishable from surrounding liver tissue by ultrasound. As the probe is withdrawn, the tract should be cauterized to prevent bleeding. A CT or magnetic resonance imaging (MRI) should be performed at 6 weeks following ablation to evaluate for technical adequacy of tumor response to ablation and thus determine whether other treatments are required.
Expected outcomes
Primary liver cancer
The most substantial number of clinical studies utilizing ablation for liver tumors have been performed for patients with HCC. Large cohort, retrospective analyses have demonstrated that ablation can achieve long term, durable, complete tumor responses. This efficacy decreases for tumors greater than 3.0 cm in size (3,6). When controlled for other risk factors such as burden of liver disease, HCC patients with tumors less than 3.0 cm in size can experience a substantial survival benefit (2,7). However, patients with tumors over 3.0 cm in size experience a survival rate of 51.5% vs. 61.4% for tumors less than 3.0 cm at 5 years (2) due to increased rate of incomplete ablation (3). Incomplete ablation drops median survival from greater than 60 months down to 31.1 months (6). Therefore, guidelines do not support attempts at ablation for tumors greater than 3.0 cm (8). In lieu of these findings, recent experimental data suggest increased aggressiveness and metastatic potential for incompletely ablated tumors due to localized damage response signaling within the tumor microenvironment (9). While randomized controlled trials of ablation vs. resection favor resection in terms of outcome (2) with a 5-year survival of 61% vs. 82%, respectively, these trials also demonstrate the significant efficacy for ablation. Initial and prolonged complete responses are thus possible following ablation (Figure 1). Thus, ablation is recommended for patients that are not resection candidates as part of standard of care guidelines for the treatment of small HCCs as defined by the American Association for the Study of Liver Diseases (AASLD) and the Barcelona Clinic Liver Cancer (BCLC) criteria (8,10). Indeed, recent studies to evaluate the potential for adjuvant therapy to extend survival following “curative” treatment, both ablation and resection patients were considered eligible for these randomized controlled clinical trials (11,12). However, evidence from the most recent STORM trial demonstrated a favorability of resection over ablation with a median recurrence free survival of 38.7–41.7 months for resection vs. 19.6–22.1 months for ablation, thus confirming ablation as a secondary modality to resection for HCC (11). Adjuvant sorafenib did not provide any additional benefit to ablation alone in this randomized, controlled trial. Additionally, ablation has been utilized successfully for several years as a means to “down-stage” HCC burdens or “bridge” patients for liver transplant candidacy (13).
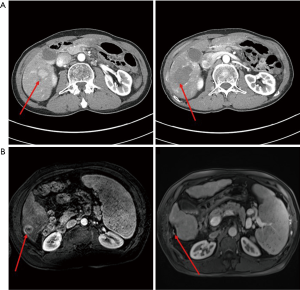
Cholangiocarcinoma (CCA) is the 2nd most common, but much more rare in incidence, primary liver cancer and thus is less extensively studied with respect to ablation. However, ablation has been utilized, primarily for intrahepatic CCAs. Since intrahepatic CCAs often present at sizes much larger than 3 cm, other treatment approaches such as resection are more often considered for initial treatment. However, efficacy has been demonstrated for ablation primarily in the setting of intrahepatic metastatic recurrences following initial resection with a 5-year overall survival of 23.7% following ablation for recurrence (14). Thus, ablation can provide significant tumor responses and increase overall survival in the absence of chemotherapy for patients with CCA.
Hepatic metastases
Ablation has been used for a variety of hepatic metastases with significant efficacy, however the most extensively studied has been for the treatment of colorectal adenocarcinoma metastases (CRMs) due to the higher incidence of these patients available for analysis compared to patients with other cancer metastases. Similar to the results with HCC, ablation has been shown to be efficacious for CRM patients, particularly when tumor sizes are less than 3.0 cm (1). While no randomized controlled trials comparing resection and ablation have been performed, case control retrospective studies strongly suggest superior outcomes for resection over ablation with overall survival at 4 years of 65% for resection vs. 22% for ablation (5). CRMs harboring RAS mutations have worse outcome with both higher local recurrence rate and lower progression free survival rate at 3 years of 35% for mutant vs. 71% for wild type (15). Additionally, ablation has been used in combination with resection during single stage operations or as part of a 2-stage approach to clear one hepatic lobe of disease followed by contralateral hepatic lobectomy at a later stage operation for treatment of larger volume of tumor burden (16). Studies in CRMs patients are often difficult to interpret secondary to use of effective adjuvant chemotherapy regimens that are often incompletely defined in ablation studies, however significant evidence suggests a benefit to use of ablation for patients who are otherwise not a candidate for hepatic resection.
Complications
In properly selected patients, morbidity and mortality rates are less than 1% collectively across selected series (17,18). Side effects such as pain, low grade fevers, and transient elevation of liver function tests are common and easily treated with very little consequence. More serious, direct complications that can occur include hepatic insufficiency, biloma, biliary fistula, biliary stricture, hepatic abscess, hemorrhage, pseudoaneurysm, pleural effusion, pneumothorax, and direct injury to other structures such as the gall bladder, diaphragm or bowel. Of these, hepatic abscess is the most frequent with the risk being elevated in patients with prior or concomitant biliary-enteric anastomosis or biliary stenting being performed.
Conclusions
Laparoscopic radiofrequency ablation or microwave ablation offers the potential for curative treatment in properly selected patients with liver tumors. Ablation should be considered in patients who are otherwise not candidates for hepatic resection of liver tumors. A laparoscopic surgical ablative approach is typically chosen for patients who are not candidates for percutaneous approaches. Outcomes demonstrating significant efficacy are best studied in patients with HCC or CRMs with low complication rates, however ablation is increasingly successfully utilized in patients with other malignancies in the liver such as CCA and other metastatic tumors.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (H. Leon Pachter) for the series “Laparoscopic Surgery of the Liver and Spleen” published in Laparoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Consultant, Bristol-Myers Squibb.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang LJ, Zhang ZY, Yan XL, et al. Radiofrequency ablation versus resection for technically resectable colorectal liver metastasis: a propensity score analysis. World J Surg Oncol 2018;16:207. [Crossref] [PubMed]
- Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2010;252:903-12. [Crossref] [PubMed]
- Lu MD, Xu HX, Xie XY, et al. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: a retrospective comparative study. J Gastroenterol 2005;40:1054-60. [Crossref] [PubMed]
- Liang P, Dong B, Yu X, et al. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology 2005;235:299-307. [Crossref] [PubMed]
- Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004;239:818-25; discussion 825-7. [Crossref] [PubMed]
- Lam VW, Ng KK, Chok KS, et al. Incomplete ablation after radiofrequency ablation of hepatocellular carcinoma: analysis of risk factors and prognostic factors. Ann Surg Oncol 2008;15:782-90. [Crossref] [PubMed]
- Pompili M, Saviano A, de Matthaeis N, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol 2013;59:89-97. [Crossref] [PubMed]
- Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016;150:835-53. [Crossref] [PubMed]
- Tan L, Chen S, Wei G, et al. Sublethal heat treatment of hepatocellular carcinoma promotes intrahepatic metastasis and stemness in a VEGFR1-dependent manner. Cancer Lett 2019;460:29-40. [Crossref] [PubMed]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16:1344-54. [Crossref] [PubMed]
- Jimenez Exposito MJ, Akce M, Montero Alvarez JL, et al. CA209-9Dx: phase III, randomized, double-blind study of adjuvant nivolumab vs. placebo for patients with hepatocellular carcinoma (HCC) at high risk of recurrence after curative resection or ablation. Ann Oncol 2018;29:viii205-70.
- Yao FY, Hirose R, LaBerge JM, et al. A prospective study on downstaging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl 2005;11:1505-14. [Crossref] [PubMed]
- Xu C, Li L, Xu W, et al. Ultrasound-guided percutaneous microwave ablation versus surgical resection for recurrent intrahepatic cholangiocarcinoma: intermediate-term results. Int J Hyperthermia 2019;36:351-8. [Crossref] [PubMed]
- Odisio BC, Yamashita S, Huang SY, et al. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. Br J Surg 2017;104:760-8. [Crossref] [PubMed]
- Regimbeau JM, Cosse C, Kaiser G, et al. Feasibility, safety and efficacy of two-stage hepatectomy for bilobar liver metastases of colorectal cancer: a LiverMetSurvey analysis. HPB (Oxford) 2017;19:396-405. [Crossref] [PubMed]
- Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 2003;226:441-51. [Crossref] [PubMed]
- Xu HX, Xie XY, Lu MD, et al. Ultrasound-guided percutaneous thermal ablation of hepatocellular carcinoma using microwave and radiofrequency ablation. Clin Radiol 2004;59:53-61. [Crossref] [PubMed]
Cite this article as: Welling TH. Laparoscopic radiofrequency ablation (RFA) of liver tumors. Laparosc Surg 2020;4:3.


