Giant retroperitoneal liposarcoma incidentally discovered during bariatric surgery
Introduction
As the obesity epidemic continues to be one of the most serious public health crises in the U.S., the demand for bariatric surgical procedures has never been greater. According to the Centers for Disease Control and Prevention, 39.8% of adults 20 years and older in the U.S. were considered obese in 2015–2016 (1). By 2000, the number of bariatric procedures performed in the U.S. had gradually increased to about 37,000 annually; however, with improved surgical technique and increasing demand that number grew exponentially to 158,000 in 2011 and 228,000 in 2017 (2).
In addition to many well-established co-morbidities, obesity is also associated with a higher risk of many types of cancer (3-6). Although the rate of malignancy is higher in obese patients, there are no guidelines to screen patients for intra-abdominal neoplasms before bariatric surgery (3). Benign, pre-malignant, and malignant masses have on occasion been encountered for the first time during bariatric procedures (3,4). Greenbaum et al. (3) suggests this may be because obesity can hinder cancer screening as physical examination is more difficult (3). Imaging including ultrasound, CT, and MRI are also less sensitive on obese patients allowing pathology to go potentially unnoticed before bariatric surgery (3). Here we present a case of a patient undergoing a planned bariatric procedure, during which a 10.2 kg liposarcoma was discovered intraoperatively. To our knowledge this is the first case in the literature of a giant liposarcoma being initially discovered during a bariatric procedure.
Case presentation
The patient was a 53-year-old male with a past medical history of morbid obesity with a BMI at its highest peaking at 51.7 kg/m2, diabetes mellitus type 2, essential hypertension, hyperlipidemia, and carotid artery disease who opted to undergo Roux-en-y gastric bypass (RYGB) surgery. At presentation to the program, the patient weighed 340 lbs, but during the required pre-operative lifestyle management program he reduced his weight to 295 lbs with a BMI of 44.6 kg/m2 by the day of surgery. Pre-operative review of systems and physical examination were considered within normal expectations, with no abdominal mass palpated. Minimally-invasive RYGB was planned.
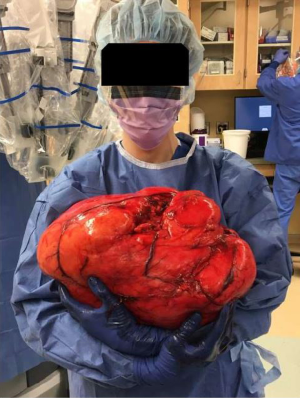
In the operating room, the patient was put under general anesthesia without event. Once the abdominal musculature was paralyzed, palpation of his abdomen detected a unilateral firmness of the right upper and lower quadrants. An upper endoscopy noted the duodenum did not sweep as expected, raising concern for either a malrotation or abdominal mass effect. Abdominal access was achieved with a visual port and four laparoscopic ports. Visual inspection revealed a translocated right colon with ileocecal valve and appendix located in the left lower quadrant. The ascending colon was carefully followed to the hepatic flexure in the normal location. At this point when mobilizing the ascending colon, a giant retroperitoneal mass was visualized. The decision was made to abort the gastric bypass and proceed with laparoscopic retroperitoneal mass excision. Dissection revealed a giant, mostly mobile tumor that did not appear to be infiltrating surrounding tissue. A Pfannenstiel incision was made for hand-assisted laparoscopic mobilization, which also verified the substantial size of the mass. An upper midline incision from xiphoid to above the umbilicus was needed to extract the mass. After extraction the tumor was measured at 41 cm and 10.2 kg (See Figures 1,2).
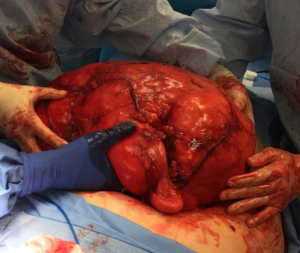
Intraoperative consultation with pathology determined it would not be possible to rule out malignancy on gross examination. Given the potential need for eventual chemotherapy or radiation it was decided to not proceed with RYGB at this time. The patient tolerated the procedure well and was discharged from the hospital on post-operative day 1 without complication.
At his post-op follow-up ten days later the patient was doing well, his surgical incisions were healing, and his weight was down to 270 lbs. When asked whether he had noticed any unusual sensation or asymmetry of his abdomen before surgery, the patient admitted that in retrospect he noticed a firmness but had assumed it was due to abdominal wall musculature secondary to increased exercise. The patient continued to recover in subsequent months with no complications from this surgery. At a subsequent multidisciplinary sarcoma tumor board conference where this patient’s case was presented, support for aborting the originally planned RYGB to manage the neoplasm was favored with recommendations to further delay bariatric and other abdominal surgeries during the surveillance period in an effort to preserve a clear radiographic picture of the retroperitoneum in the event of unresected or early recurrent disease.
Histopathological findings
Diagnosis: a well-differentiated, partly-encapsulated liposarcoma measuring 41.0×38.0×16.5 cm and weighting 10.2 kg (22.45 lbs).
Sectioning revealed lobules of dense fatty surface with yellow-white areas indicative of fat necrosis and dystrophic calcifications. The lobules contained mature adipocytes, collagenous bands, stromal cells with degenerative type atypia (loch-kern cells), and stromal cells with hyperchromatic nuclei. Fluorescence in situ hybridization demonstrated MDM2 amplification.
Discussion
Liposarcomas are malignant tumors arising from adipose cells that most frequently occur in or after middle age with equal incidence in both genders (7-9). They are particularly striking due to their potential to grow into some of the largest tumors encountered. Often they are discovered incidentally as large, painless masses, but if left to grow undetected can become symptomatic with abdominal pain or distension, early satiety, constipation, and urinary obstruction (7). Causes of soft tissue tumors are unknown but known risk factors include obesity, occupational chemical exposures, immunosuppression, radiation, and family history (8). Surgery is considered the treatment of choice and can be curative (7,9). While there is some support for radiation as adjuvant therapy, chemotherapy remains controversial (9).
Soft tissue sarcomas are rare in the adult population making up only 1% of all tumors (10). Liposarcomas are the largest sub-category of soft tissue sarcomas and are the most frequent diagnosis of retroperitoneal tumors (12–40%) (10). The behavior of liposarcomas varies greatly from well-differentiated, non-invading masses considered benign to more aggressive, invading, poorly differentiated cancers that have the potential to metastasize. Liposarcomas can arise anywhere adipose tissue collects but tend to have a significantly worse prognosis when arising in the retroperitoneum (7,10). The retroperitoneum provides a large potential space for liposarcomas to grow undetected, as was the case in our patient.
Thway et al. (7) compare well-differentiated liposarcomas (WDL) and dedifferentiated liposarcomas (DDL), noting WDL are comprised of mature adipocytes with various atypia and are generally benign lacking the ability to metastasize (7). DDL can arise de novo (90%) or as a reoccurrence after WDL resection (10%) (7). DDL have a heterogenous histopathology and are more aggressive than WDL due to a higher rate of local recurrence and ability to metastasize (7). Although the liposarcoma found in our patient was determined to be a WDL, given the potential for recurrence or transformation to DDE, he will continue to be followed by oncology for many years to come.
Mortality rate of liposarcomas varies drastically by location from essentially 0% in extremities to up to 80% when occurring in the retroperitoneum or when invading viscera (8). The actuarial survival rate for those who undergo gross resection of a primary retroperitoneal sarcoma is 51% at 5 years and 36% at 10 years (9,11).
The incidence of unexpected pathology found during bariatric procedures is reported to be between 1.5–8% (3,5,12). Finnell et al. (12) reviewed 398 cases of two bariatric surgeons noting 2% of all cases found unexpected pathologic lesions during surgery (12). Greenbaum et al. (3) reviewed 400 bariatric cases, 8% of which encountered unexpected lesions, the majority (80%) arising from the ovary and only 3 malignant neoplasms (3). Walędziak et al. (5) reviewed 1,252 bariatric surgical cases done by one surgical team reporting 3.9% of cases were positive for a variety of abnormalities, including 1.28% with GISTs, 0.40% with leiomyomas, 0.24% with lipomas, 0.16% with fibromas, 0.16% with Schwannomas, and 0.08% with neurofibromas (5). These studies further support the observation that obesity increases the risk of developing a variety of neoplasms.
Given their potential for great size, liposarcomas tend to be described in the literature as masses easily found on physical exam for which a subsequent surgery is scheduled to resect. Moyon et al. (10) described the case of a giant 40×28×10 cm abdominal liposarcoma harbored by a patient for 2 years after initially detected due to lack of healthcare (10). At the time of resection, the patient not only had a palpable and painful mass but she appeared malnourished and was otherwise symptomatic. Akhoondinasab et al. (9) reported a 32 kg liposarcoma in a patient who had become emaciated before tumor removal who also delayed surgical resection for several years after detection (9). At one-year post-op there were two areas of recurrence which were surgically removed. Another 2 years of monitoring had no new recurrences. Our patient was unique in that the liposarcoma, although of great size, was only found incidentally during bariatric surgery. This is an example of how pathology can go unnoticed in the case of confounding body habitus. To our knowledge this is the first case in the literature of a giant liposarcoma being initially discovered during a bariatric procedure.
An alternative to the surgical approach taken in this case would have been to biopsy the incidental mass and conclude the procedure without resection. Biopsy is often used intraoperatively to guide surgical decision making and is of great value especially when the identity of an incidental mass is not certain. However, in this case, once the incidental mass was visualized and analyzed, intraoperative consultation with pathology determined high likelihood given the characteristics and size of the mass that it was a lipoma or liposarcoma. Given the immense proportions of the mass, there was concern biopsy would not reflect all varying components within the large mass and there would be a high degree of sampling error. Since the mass was well-encapsulated – pushing structures rather than invasion – and anticipating the inevitability of resection, we proceeded with resection. This is not an uncommon approach when clinical suspicion is high. Two separate retrospective analyses conducted by Finnell et al. (12) and Joo et al. (13) illustrate this, that unexpected findings are relatively common in bariatric surgery and that changes to the original surgical plan – including adding non-bariatric procedures – are necessary and not associated with increases in complications or morbidity (12,13). Finnell et al. (12) found that the unexpected pathology was removed without biopsy in 87.5% of cases during the bariatric procedure, and that these cases were not associated with postoperative complications (12). Resection of masses based on clinical judgement rather than biopsy is found in other surgical fields aside from bariatrics. Levy et al. (14) determined preoperative diagnosis of hepatocellular carcinoma was highly accurate in larger lesions (>3 cm) making preoperative tumor biopsy unnecessary (14). Ozeki et al. (15) found they were able to predict with high probability whether lung masses were cancerous based only on imaging studies and were able to proceed with surgical resection without pre- or intra-operative biopsy for definitive diagnosis (15). Whether or not biopsy is done is a matter of clinical judgement. Surgery resection remains the mainstay of treatment for retroperitoneal sarcomas regardless of their state of differentiation (16).
Conclusions
Morbid obesity increases the risk of developing a variety of neoplasms (3-6). Pathology that may be detected in the average patient may go unnoticed in an obese patient during the workup for a bariatric procedure. This is in part because there are no guidelines to screen patients for intra-abdominal malignancy before bariatric surgery, and because obesity can mask even very large abnormal masses on physical exam. Understanding 1.5–8% of bariatric procedures encounter abnormal pathology, in addition to a careful physical exam in the office, a reasonable addition may be a second, thorough abdominal exam when the abdominal musculature has been relaxed prior to incision. Surgeons should be prepared to abort the bariatric procedure to manage an incidentally discovered neoplasm.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Dr. Yarbrough has been compensated with a stipend from Intuitive Surgical for proctoring and teaching robotic surgery. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- 1. Centers for Disease Control and Prevention. Obesity and overweight. Available online: https://www.cdc.gov/nchs/fastats/obesity-overweight.htm. Accessed April 3, 2019.
- American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2017. Available online: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed March 27, 2019.
- Greenbaum D, Friedel D. Unanticipated findings at bariatric surgery. Surg Obes Relat Dis 2005;1:22-4. [Crossref] [PubMed]
- Raghavendra RS, Kini D. Benign, premalignant, and malignant lesions encountered in bariatric surgery. JSLS 2012;16:360-72. [Crossref] [PubMed]
- Walędziak M, Różańska-Walędziak A, Kowalewski PK, et al. Bariatric surgery and incidental gastrointestinal stromal tumors - a single-center study: VSJ Competition, 1st place. Wideochir Inne Tech Maloinwazyjne 2017;12:325-9. [Crossref] [PubMed]
- Steele CB, Thomas CC, Henley SJ, et al. Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity - United States, 2005-2014. MMWR Morb Mortal Wkly Rep 2017;66:1052-8. [Crossref] [PubMed]
- Thway K, Jones RL, Noujaim J, et al. Dedifferentiated Liposarcoma: Updates on Morphology, Genetics, and Therapeutic Strategies. Adv Anat Pathol 2016;23:30-40. [Crossref] [PubMed]
- Matone J, Okazaki S, Maccapani GN, et al. Giant gastric lipossarcoma: case report and review of the literature. Einstein (Sao Paulo) 2016;14:557-60. [Crossref] [PubMed]
- Akhoondinasab MR, Omranifard M. Huge retroperitoneal liposarcoma. J Res Med Sci 2011;16:565-7. [PubMed]
- Moyon FX, Moyon MA, Tufiño JF, et al. Massive retroperitoneal dedifferentiated liposarcoma in a young patient. J Surg Case Rep 2018;2018:rjy272 [Crossref] [PubMed]
- Hassan I, Park SZ, Donohue JH, et al. Operative management of primary retroperitoneal sarcomas: a reappraisal of an institutional experience. Ann Surg 2004;239:244-50. [Crossref] [PubMed]
- Finnell CW, Madan AK, Ternovits CA, et al. Unexpected pathology during laparoscopic bariatric surgery. Surg Endosc 2007;21:867-9. [Crossref] [PubMed]
- Joo P, Guilbert L, Sepúlveda EM, et al. Unexpected Intraoperative Findings, Situations, and Complications in Bariatric Surgery. Obes Surg 2019;29:1281-6. [Crossref] [PubMed]
- Levy I, Greig PD, Gallinger S, et al. Resection of hepatocellular carcinoma without preoperative tumor biopsy. Ann Surg 2001;234:206-9. [Crossref] [PubMed]
- Ozeki N, Iwano S, Taniguchi T, et al. Therapeutic surgery without a definitive diagnosis can be an option in selected patients with suspected lung cancer. Interact Cardiovasc Thorac Surg 2014;19:830-7. [Crossref] [PubMed]
- Mullen JT, DeLaney TF. Clinical features, evaluation, and treatment of retroperitoneal soft tissue sarcoma. 2018. UpToDate.com. Available online: https://www.uptodate.com/contents/clinical-features-evaluation-and-treatment-of-retroperitoneal-soft-tissue-sarcoma#H14. Accessed July 16, 2019.
Cite this article as: Calderon H, Yarbrough D, La Vella E. Giant retroperitoneal liposarcoma incidentally discovered during bariatric surgery. Laparosc Surg 2019;3:36.