
Near-infrared intraoperative imaging of pancreatic neuroendocrine tumors: a new era in laparoscopic pancreatic surgery
It is evident that safe complete resection of the target tumor without excess or deficiency is the most important object in the oncological surgery, besides this is particularly true in the laparoscopic surgery for pancreatic neuroendocrine tumors (PanNETs). In the recent study by Paiella et al. (1) the near-infrared (NIR) technology using indocyanine green (ICG) as an NIR fluorophore was tested in laparoscopic resection of PanNETs. The authors reported that PanNETs are intraoperatively visualized as brighter glow against background normal pancreatic tissue. They have applied a light with specific wave length around 800 nm to the ICG dye that was intravenously injected on site in the operation room, and found that 10 out of 10 PanNETs were visually identified. So, they could have done nine laparoscopic distal pancreatectomies and one laparoscopic enucleation safely. This is the first application of NIR to the PanNET surgery. Because the treatment of PanNETs sometimes bothers surgeons, this method may become a new standard in laparoscopic resection of PanNETs.
Treatment of PanNETs requires three important procedures; first, securing the presence of tumors responsible for the symptoms, second, localizing the tumors and visualizing them if possible, and third, oncologically complete resection of the tumors. Owing to the recent improvement of imaging techniques, increasing number of PanNETs are incidentally found. PanNETs consist of functioning PanNETs which express various functional syndrome and non-functioning PanNETs which do not express distinct clinical syndrome (2). Insulinoma is the most common functioning PanNET and other functioning PanNETs are rare. Recent epidemiological study showed that 47.4% of all PanNETs was non-functioning PanNETs (3).
In the study by Paiella et al. (1), 20% of the PanNETs was insulinoma. Insulinoma is located in the pancreas and causes a characteristic syndrome due to hypoglycemia caused by excessive insulin from the tumor (4). Because of these symptoms, it may be easy to predict the presence of insulinoma. Because most of insulinomas are located entirely within the pancreas, they can be visualized by US, CT and MRI. Especially, EUS has a great sensitivity and specificity for intra-pancreatic PanNETs. Inversely, the somatostatin receptor scintigraphy (SRS) has a lower detection rate on insulinomas because insulinomas do not have enough densities of somatostatin receptors; therefore more than half of insulinomas are not found by preoperative SRS (5).
When the tumor is solitary, it is highly possible that the tumor is responsible for the symptoms. However, it is sometimes difficult to find out which tumor is responsible for the hypoglycemia, because approximately 10% of insulinomas are multiple, and about 5% are related with the multiple endocrine neoplasia type 1 (MEN1) syndrome (6). In such cases, we can determine where the responsible tumor exists preoperative by the intra-arterial calcium injection test (SACI test) with hepatic venous insulin sampling (7,8). Along with these processes, we can make it possible to decide which tumor or which part of the pancreas should be removed.
Because of the serious symptoms such as headache, confusion, visual disturbance, sweating, tremor and palpitation, surgical resection is recommended once diagnosed. Isolated sporadic insulinoma can be cured by pancreatic resection (4,6). The purpose of surgical resection is to cure the symptoms caused by an excessive insulin by removing all insulinomas. Generally, lymph node dissection is not required for insulinoma surgery (9). Since MEN1 patients often have other PanNETs which are non-functioning tumors, preoperative accurate localization of functioning tumor is crucial (10).
Another target in the study by Paiella et al. (1) was non-functioning PanNETs. Final pathology in their study showed 80% of the resected tumors was non-functioning PanNETs. Thanks to the recent imaging technologies, the number of non-functioning PanNETs is increasing, but these tumors do not express clinical symptoms. Therefore, even if you see the tumor on US or CT image, it is difficult to make a quick decision of surgical resection.
Patients with malignant tumors should be operated on aggressively, while the surgical risk-benefit ratio could be carefully waited in possibly benign tumors smaller than 2 cm. Most of PanNETs with a diameter less than 2 cm are likely benign; however, regarding incidentally discovered non-functioning PanNETs, 6% are malignant even if they are smaller than 2 cm (11). Presently, no agreement exists on the diameter cutoff. Actually, Gratian et al. (12) reported that 29% and 10% of non-functioning PanNETs smaller than 2cm had lymph node metastases and distant metastases, respectively. Although the malignant potential cannot be excluded completely, a 2-cm cutoff is considered sufficiently safe so far (13). Finally, non-functioning PanNETs with a diameter bigger than 2 cm and with a yearly increased diameter more than 0.5 cm should be the candidate for surgical resection.
Whether the target is an insulinoma or a non-functioning PanNET, what you have to do is a complete resection without any residual. When you choose open surgery, it will be not difficult to identify the target tumor in the pancreas by intraoperative US or palpation. Tumors in the head of pancreas are treated with a pancreaticoduodenectomy and tumors of the body and tail are treated with a distal pancreatectomy with or without spleen preservation. The disadvantages of typical pancreatic resections are a high incidence of perioperative complications and long-term exocrine and endocrine insufficiency (14).
Concerning the management of non-functioning PanNETs, in particular when the boundary is clear and the size is small, atypical resection is proposed. Middle pancreatectomy is performed only on small tumors of the pancreas and enucleation is done only when the main pancreatic duct is sufficiently far from the tumor. The most important advantage of atypical resection is a reduction in long-term endocrine and exocrine insufficiency compared to typical resections (15). On the other hand, the influence of atypical resection is almost temporary and clinical impact is minimal, but it sometimes causes pancreatic fistula. Furthermore, it is difficult to obtain an oncological negative margin by enucleation, and lymph node dissection is not performed; thus, only local lymph node sampling is done. Therefore, atypical resection should be considered only for small lesions with benign or uncertain behavior.
Recently, the number of laparoscopic surgery is increasing when PanNETs can be localized preoperatively (16). When preoperative localization of the tumors is completed, laparoscopic enucleation or laparoscopic pancreatectomy can be performed (17). If the diameter of the tumor is less than 2 cm and located further than 3 mm from the main pancreatic duct, a laparoscopic enucleation is considered rather than pancreatic resection. Inversely, when the tumor is not far enough from the main pancreatic duct, enucleation should be avoided and a laparoscopic pancreatectomy such as distal pancreatectomy is considered. In any case, intraoperative precise localization of the tumor is extremely important.
When laparoscopic distal pancreatectomy is planned, it would be enough to know the approximate localization of the PanNET. Then intraoperative US and simultaneous marking with dye or with an energy device will work. However, in case of laparoscopic enucleation of PanNET, real-time identification of the tumor is indispensable.
Paiella et al. (1) introduced the NIR technology using ICG in the field of laparoscopic pancreatic surgery. Among many NIR fluorescent molecules ICG is most popularly used. Since Kitai (18) first performed fluorescent navigation of sentinel lymph nodes using ICG and light emitting diodes, the intraoperative use of near-infrared fluorescence has served an important role in increasing our understanding in a variety of fields such as surgical oncology and ocular, cardiocirculatory, and liver function diagnostics. Recent reports regarding intraoperative sentinel lymph node mapping and HCC detection have further extended its clinical application.
Near-infrared light can penetrate human tissues to a depth up to 4 cm; however, it is greatly affected by the overlying tissue thickness (19,20). This is true for both the excitation and emission wavelengths of ICG. Therefore, clinically a penetration depth of only several millimeters with low fidelity can be achieved (21,22). If the target stays within several millimeters depth, NIR fluorophores may provide higher sensitivity for intraoperative and non-invasive imaging due to their capability to be repeatedly excited by tissue-penetrating NIR excitation light. ICG is a tricarbocyanine dye that has been clinically used for over 50 years for hepatic clearance, cardiovascular function test, and retinal angiography, typically administered at concentrations of 2.5 mg/mL at typical total doses of 25 mg in adults. ICG does associate with albumin, making it an excellent vascular agent for evaluating both the blood and lymph systems. Because ICG absorb the light waves around 800 nm and emit light with peak emission at 835 nm, the ICG can be visualized on the monitor through a special CCD camera, collimator. We can detect blood and lymphatic vessels by the fluorescence of ICG.
Paiella et al. (1) studied nine cases of laparoscopic distal pancreatectomy and one laparoscopic enucleation. As discussed above, laparoscopic distal pancreatectomy does not necessarily require real-time NIR imaging because approximate position of the tumor is preoperatively determined. Furthermore, an addition of intraoperative US will help a lot. Of course by the real-time NIR imaging it will become easier to determine the cut line keeping sufficient margin from the tumor.
The real-time NIR imaging is most useful for laparoscopic enucleation of PanNETs. The authors found that ICG is accumulated in PanNETs, and emission can be visualized by the operator so they could recognize the localization of the tumor. This is a new idea of navigation surgery for PanNETs. They described that all the 10 tumors could be seen as brighter area than surrounding normal pancreas tissue, and surgeons could easily determine the location of PanNETs; however, they did not discuss why PanNETs showed stronger fluorescence.
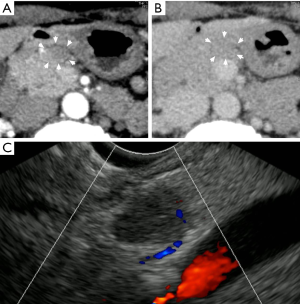
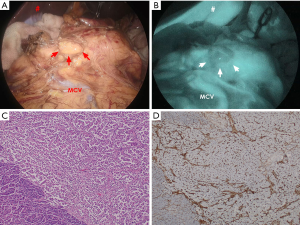
We speculate that the intensity of emitted fluorescence just reflects the blood flow within the tumor. Paiella et al. tested ICG- NIR imaging on two insulinomas and 8 non-functioning PanNETs in their series (1). Generally PanNET is believed to have high vascularity. So we tried to test ICG- NIR imaging for PanNET which was thought to have low vascularity based on preoperative US and CT. The subject was a non-functioning PanNET of a 51-year-old female. By contrast CT, the tumor was not obviously enhanced neither in the arterial phase nor the equilibrium phase, and the blood flow in the tumor was not observed by Doppler US (Figure 1). The tumor was not definitively seen by the laparoscopic light source (Figure 2A); then ICG was intravenously administered to the patient according to the method of Paiella et al. (1). After 200 seconds, the tumor was detected as a mass with lower fluorescence intensity against the surrounding pancreas (Figure 2B). Histologically, this tumor was a non-functioning PanNET (Figure 2C) with normal staining by CD34 antibody (Figure 2D).
By the Paiella's research and our case, it is proved that ICG-NIR imaging can clearly visualize the localization of PanNETs at real-time during surgical procedure. Whether the tumor is seen brighter or darker, this method is particularly useful for laparoscopic enucleation of PanNETs. Paiella et al. (1) suggested that the administration method of ICG is suitable and further suggested that bolus administration of ICG will be good enough rather than divided doses of ICG. As presented above, however, PanNETs show various range of vascularity. It should be necessary to examine the optimal dose and timing of ICG administration for PanNETs with different vascularity presumed by US and CT.
In conclusion, their new attempt in practice is highly appreciated. The new method of visualizing tumors will exert power in laparoscopic tumor enucleation for PanNETs. It will also have an important role in determining the cut-line in laparoscopic distal pancreatectomy. The method will be most practical if the protocol is more simplified.
Acknowledgments
The authors gratefully appreciate the comments on pathological findings provided by Doctors Tomoyuki Shirase and Tomoko Okuno, Department of Pathology, Japanese Red Cross Otsu Hospital, and the clinical assistance provided by Doctors Hideaki Oe, Katsuaki Ura, Yusuke Nakayama, Jun Matsubayashi, Akihiro Kaneda, Takumi Kozu, Department of Surgery, Japanese Red Cross Otsu Hospital.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Laparoscopic Surgery. The article did not undergo external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ls.2018.05.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Paiella S, De Pastena M, Landoni L, et al. Is there a role for near-infrared technology in laparoscopic resection of pancreatic neuroendocrine tumors? Results of the COLPAN "colour-and-resect the pancreas" study. Surg Endosc 2017;31:4478-84. [Crossref] [PubMed]
- Kulke MH, Anthony LB, Bushnell DL, et al. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas 2010;39:735-52. [Crossref] [PubMed]
- Ito T, Sasano H, Tanaka M, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol 2010;45:234-43. [Crossref] [PubMed]
- Guettier JM, Gorden P. Insulin secretion and insulin-producing tumors. Expert Rev Endocrinol Metab 2010;5:217-27. [Crossref] [PubMed]
- Mathur A, Gorden P, Libutti SK. Insulinoma. Surg Clin North Am 2009;89:1105-21. [Crossref] [PubMed]
- Zhao YP, Zhan HX, Zhang TP, et al. Surgical management of patients with insulinomas: Result of 292 cases in a single institution. J Surg Oncol 2011;103:169-74. [Crossref] [PubMed]
- Kato M, Doi R, Imamura M, et al. Calcium-evoked insulin release from insulinoma cells is mediated via calcium-sensing receptor. Surgery 1997;122:1203-11. [Crossref] [PubMed]
- Doi R, Komoto I, Nakamura Y, et al. Pancreatic Endocrine Tumor in Japan. Pancreas 2004;28:247-52. [Crossref] [PubMed]
- Ellison EC, Johnson JA. The Zollinger-Ellison syndrome: a comprehensive review of historical, scientific, and clinical considerations. Curr Probl Surg 2009;46:13-106. [Crossref] [PubMed]
- Jensen RT, Berna MJ, Bingham DB, et al. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer 2008;113:1807-43. [Crossref] [PubMed]
- Bettini R, Partelli S, Boninsegna L, et al. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery 2011;150:75-82. [Crossref] [PubMed]
- Gratian L, Pura J, Dinan M, et al. Impact of extent of surgery on survival in patients with small nonfunctional pancreatic neuroendocrine tumors in the United States. Ann Surg Oncol 2014;21:3515-21. [Crossref] [PubMed]
- Falconi M, Zerbi A, Crippa S, et al. Parenchyma-preserving resections for small nonfunctioning pancreatic endocrine tumors. Ann Surg Oncol 2010;17:1621-7. [Crossref] [PubMed]
- Smith JK, Ng SC, Hill JS, et al. Complications after pancreatectomy for neuroendocrine tumors: a national study. J Surg Res 2010;163:63-8. [Crossref] [PubMed]
- Falconi M, Mantovani W, Crippa S, et al. Pancreatic insufficiency after different resections for benign tumours. Br J Surg 2008;95:85-91. [Crossref] [PubMed]
- Richards ML, Thompson GB, Farley DR, et al. Setting the bar for laparoscopic resection of sporadic insulinoma. World J Surg 2011;35:785-9. [Crossref] [PubMed]
- Isla A, Arbuckle JD, Kekis PB, et al. Laparoscopic management of insulinomas. Br J Surg 2009;96:185-90. [Crossref] [PubMed]
- Kitai T, Inomoto T, Miwa M, et al. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer 2005;12:211-5. [Crossref] [PubMed]
- Marshall MV, Rasmussen JC, Tan IC, et al. Near-Infrared Fluorescence Imaging in Humans with Indocyanine Green: A Review and Update. Open Surg Oncol J 2010;2:12-25. [Crossref] [PubMed]
- Feng W, Haishu D, Fenghua T, et al. Influence of overlying tissue and probe geometry on the sensitivity of a near-infrared tissue oximeter. Physiol Meas 2001;22:201-8. [Crossref] [PubMed]
- Miyata A, Ishizawa T, Kamiya M, et al. Photoacoustic tomography of human hepatic malignancies using intraoperative indocyanine green fluorescence imaging. PLoS One 2014;9:e112667 [Crossref] [PubMed]
- Ishizawa T, Fukushima N, Shibahara J, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 2009;115:2491-504. [Crossref] [PubMed]
Cite this article as: Doi R, Toyoda E, Kitaguchi K, Abe Y, Hirose T. Near-infrared intraoperative imaging of pancreatic neuroendocrine tumors: a new era in laparoscopic pancreatic surgery. Laparosc Surg 2018;2:29.


